Big Formulas
... SO2 Ca(OH )2 CaSO3 H 2O : Formula that represents the process of “scrubbing” products of industrial combustion processes. Sulfur dioxide gas is removes by using an aqueous solution of calcium hydroxide, also called limewater. The sulfur dioxide reacts with the limewater to form solid calcium s ...
... SO2 Ca(OH )2 CaSO3 H 2O : Formula that represents the process of “scrubbing” products of industrial combustion processes. Sulfur dioxide gas is removes by using an aqueous solution of calcium hydroxide, also called limewater. The sulfur dioxide reacts with the limewater to form solid calcium s ...
I Must Have That Formula APES Chemistry Review From Kelly A
... Another process for scrubbing that utilizes magnesium hydroxide instead of limewater. The sulfur dioxide dissolves in the water and reacts with the magnesium hydroxide to form a salt. The magnesium sulfite that is formed can be isolated and heated to regenerate sulfur dioxide. The recovered sulfur d ...
... Another process for scrubbing that utilizes magnesium hydroxide instead of limewater. The sulfur dioxide dissolves in the water and reacts with the magnesium hydroxide to form a salt. The magnesium sulfite that is formed can be isolated and heated to regenerate sulfur dioxide. The recovered sulfur d ...
Radioactivity - Teach Nuclear
... Isotopes have different but similar properties, e.g., ordinary water (water with atoms) boils at 1000 C but heavy water (water with atoms) boils at 101.420 C ...
... Isotopes have different but similar properties, e.g., ordinary water (water with atoms) boils at 1000 C but heavy water (water with atoms) boils at 101.420 C ...
Stoichiometry
... The charge on a compound must net zero. The charges on the representative elements are predictable; ...
... The charge on a compound must net zero. The charges on the representative elements are predictable; ...
W. M. White Geochemistry Chapter 9: Stable Isotopes Chapter 9
... The elements interest in stable isotope geochemistry are H, Li, B, C, N, O, Si, S, and Cl. Of these, O, H, C and S are of the greatest interest. Most of these elements have several common characteristics: (1) They have low atomic mass. (2) The relative mass difference between their isotopes is large ...
... The elements interest in stable isotope geochemistry are H, Li, B, C, N, O, Si, S, and Cl. Of these, O, H, C and S are of the greatest interest. Most of these elements have several common characteristics: (1) They have low atomic mass. (2) The relative mass difference between their isotopes is large ...
The Periodic Table, Atomic Structure, Isotopes, Ions and Nomenclature
... smaller and smaller pieces, how far could you go? • You would eventually end up with atoms (translates to “indivisible” in greek) of pure carbon. • You can not divide a carbon atom into smaller pieces and still have carbon ...
... smaller and smaller pieces, how far could you go? • You would eventually end up with atoms (translates to “indivisible” in greek) of pure carbon. • You can not divide a carbon atom into smaller pieces and still have carbon ...
Lecture 33 - Cornell Geological Sciences
... • Cosmogenic nuclides are also used to date meteorites exposure ages are much less than ‘formation’ ages, telling us meteorites come from larger bodies in which they were shielded from cosmic rays. ...
... • Cosmogenic nuclides are also used to date meteorites exposure ages are much less than ‘formation’ ages, telling us meteorites come from larger bodies in which they were shielded from cosmic rays. ...
Objective 2 Average Atomic Mass
... whole new field of research. Among those who worked in this new field were Pierre and Marie Curie. The Curies discovered that some forms of matter give off (12) ________________, a combination of particles and energy. Marie Curie named this process (13) ________________ Another term used to describe ...
... whole new field of research. Among those who worked in this new field were Pierre and Marie Curie. The Curies discovered that some forms of matter give off (12) ________________, a combination of particles and energy. Marie Curie named this process (13) ________________ Another term used to describe ...
Remediation_unit 2_standard
... whole new field of research. Among those who worked in this new field were Pierre and Marie Curie. The Curies discovered that some forms of matter give off (12) ________________, a combination of particles and energy. Marie Curie named this process (13) ________________ Another term used to describe ...
... whole new field of research. Among those who worked in this new field were Pierre and Marie Curie. The Curies discovered that some forms of matter give off (12) ________________, a combination of particles and energy. Marie Curie named this process (13) ________________ Another term used to describe ...
The nucleus Rutherford`s nuclear atom (1902
... of 'new' radioactive elements which could not be fitted into the ten or so gaps in the Periodic Table. – However, it was found that some of these elements had identical chemical properties although their radioactive properties, such as half-life and type of emitted radiation, differed. ...
... of 'new' radioactive elements which could not be fitted into the ten or so gaps in the Periodic Table. – However, it was found that some of these elements had identical chemical properties although their radioactive properties, such as half-life and type of emitted radiation, differed. ...
The nucleus - VCE Chemistry
... • The existence of isotopes could now be explained - isotopes of the same element contain the same number of protons in the nucleus but the number of neutrons may vary. • The neutron subsequently proved to be a very useful tool with which to investigate the atom. • As an uncharged particle it can ea ...
... • The existence of isotopes could now be explained - isotopes of the same element contain the same number of protons in the nucleus but the number of neutrons may vary. • The neutron subsequently proved to be a very useful tool with which to investigate the atom. • As an uncharged particle it can ea ...
The average atomic mass of an element is the sum of the
... or 4 atomic mass units), and they are called isotopes. For any given isotope, the sum of the numbers of protons and neutrons in the nucleus is called themass number. This is because each proton and each neutron weigh one atomic mass unit (amu). By adding together the number of protons and neutrons a ...
... or 4 atomic mass units), and they are called isotopes. For any given isotope, the sum of the numbers of protons and neutrons in the nucleus is called themass number. This is because each proton and each neutron weigh one atomic mass unit (amu). By adding together the number of protons and neutrons a ...
Isotopes Article
... Helium has an atomic number of 2 so Helium has two protons. Since all atoms on the Periodic Table are neutral, Helium also has two electrons. Remember, if an atom has 43 protons, it also has 43 electrons. The number of protons for a given atom of a specific element will never change. If Helium gaine ...
... Helium has an atomic number of 2 so Helium has two protons. Since all atoms on the Periodic Table are neutral, Helium also has two electrons. Remember, if an atom has 43 protons, it also has 43 electrons. The number of protons for a given atom of a specific element will never change. If Helium gaine ...
can be determined without changing the identity of matter
... We classify PROPERTIES of substances by whether or not you must change the identity of a substance to obtain information about the property ...
... We classify PROPERTIES of substances by whether or not you must change the identity of a substance to obtain information about the property ...
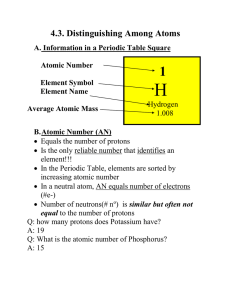
Notes 4.3 filled in
... MN is not indicated on the PT. To calculate the MN, simply add up #p+ and # no each having the mass of 1 amu. Q: an atom has 5 protons and 7 neutrons, calculate the MN. A: 5 amu + 7 amu = 12 amu Q: Which element is that? (Look in the PT) A: 5 protons, it’s Boron D. Isotopes Every element exist ...
... MN is not indicated on the PT. To calculate the MN, simply add up #p+ and # no each having the mass of 1 amu. Q: an atom has 5 protons and 7 neutrons, calculate the MN. A: 5 amu + 7 amu = 12 amu Q: Which element is that? (Look in the PT) A: 5 protons, it’s Boron D. Isotopes Every element exist ...
1412-PracticeExam4
... CH3 CH2CH3 b. trans-5-methyl-2-hexene c. Draw the following molecule: 1-ethyl-1-methylcyclopentane ...
... CH3 CH2CH3 b. trans-5-methyl-2-hexene c. Draw the following molecule: 1-ethyl-1-methylcyclopentane ...
mass number - KCPE-KCSE
... mass of one atom of carbon-12 Most elements have more than one isotope. The Ar of the element is the average mass of the isotopes taking into account the abundance of each isotope. This is why the Ar of an element is frequently not a whole number. ...
... mass of one atom of carbon-12 Most elements have more than one isotope. The Ar of the element is the average mass of the isotopes taking into account the abundance of each isotope. This is why the Ar of an element is frequently not a whole number. ...
SMP Quiz Session 1
... You have two atoms, one has 6 protons, 6 neutrons, and 6 electrons and the other has 6 protons, 8 neutrons, and 6 electrons. Which of the following best describes the difference between them? The ...
... You have two atoms, one has 6 protons, 6 neutrons, and 6 electrons and the other has 6 protons, 8 neutrons, and 6 electrons. Which of the following best describes the difference between them? The ...
4550-15Lecture29 - Cornell Geological Sciences
... • Cosmogenic nuclides are also used to date meteorites exposure ages are much less than ‘formation’ ages, telling us meteorites come from larger bodies in which they were shielded from cosmic rays. ...
... • Cosmogenic nuclides are also used to date meteorites exposure ages are much less than ‘formation’ ages, telling us meteorites come from larger bodies in which they were shielded from cosmic rays. ...
Energy per nucleon
... oxidizes to CO2 and is converted by plants into organic matter. Particularly durable are wood and charcoal generated from wood. ...
... oxidizes to CO2 and is converted by plants into organic matter. Particularly durable are wood and charcoal generated from wood. ...
File
... the box for each element (look at periodic table). • The atomic number (or number of protons) identifies an element. • The modern periodic table orders elements according to increasing atomic number. • The charge of a proton is assigned numerical value of +1. • Mass of a proton is 1.0 amu ...
... the box for each element (look at periodic table). • The atomic number (or number of protons) identifies an element. • The modern periodic table orders elements according to increasing atomic number. • The charge of a proton is assigned numerical value of +1. • Mass of a proton is 1.0 amu ...
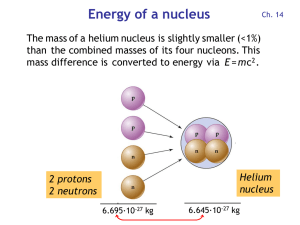
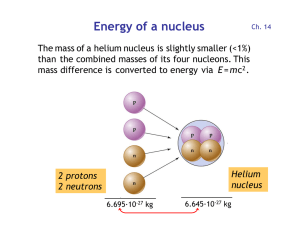
Energy of a nucleus
... oxidizes to CO2 and is converted by plants into organic matter. Particularly durable are wood and charcoal generated from wood. ...
... oxidizes to CO2 and is converted by plants into organic matter. Particularly durable are wood and charcoal generated from wood. ...
isotopes notes
... • Carbon will always have 6 protons How many neutrons does Carbon-14 have? there are 14 total protons and neutrons. 6 of them are protons. So 14 (total) – 6 (protons) = 8 Neutrons How many neutrons does Carbon-12 have? ...
... • Carbon will always have 6 protons How many neutrons does Carbon-14 have? there are 14 total protons and neutrons. 6 of them are protons. So 14 (total) – 6 (protons) = 8 Neutrons How many neutrons does Carbon-12 have? ...
Isotope analysis

Isotope analysis is the identification of isotopic signature, the distribution of certain stable isotopes and chemical elements within chemical compounds. This can be applied to a food web to make it possible to draw direct inferences regarding diet, trophic level, and subsistence. Variations in isotope ratios from isotopic fractionation are measured using mass spectrometry, which separates the different isotopes of an element on the basis of their mass-to-charge ratio.The ratios of isotopic oxygen are also differentially affected by global weather patterns and regional topography as moisture is transported. Areas of lower humidity cause the preferential loss of 18O water in the form of vapor and precipitation. Furthermore, evaporated 16O water returns preferentially to the atmospheric system as it evaporates and 18O remains in liquid form or is incorporated into the body water of plants and animals.