1970 - 2005 Solids/Liquids/Solutions FRQs
... what changes, if any, occur to bring the system to equi1 kg water = 55.6 mol water librium. ...
... what changes, if any, occur to bring the system to equi1 kg water = 55.6 mol water librium. ...
CHAPTER 4: CHEMICAL QUANTITIES and AQUEOUS REACTIONS
... 14.4 g of salicylic acid (limiting reactant) gives actual yield of 6.26 g of aspirin. Calculate the percent yield of aspirin using the following equation. ...
... 14.4 g of salicylic acid (limiting reactant) gives actual yield of 6.26 g of aspirin. Calculate the percent yield of aspirin using the following equation. ...
1994–PTAS, Inc - mvhs
... differences (and thus only certain frequencies) will result. So you see line spectra corresponding to those frequencies. 2) n = 4 allows for 4 values of L L = 0 corresponds to an s-orbital, the shape is spherical, there is 1 s-orbital L = 1 corresponds to a p-orbital, the shape is dual-lobe along th ...
... differences (and thus only certain frequencies) will result. So you see line spectra corresponding to those frequencies. 2) n = 4 allows for 4 values of L L = 0 corresponds to an s-orbital, the shape is spherical, there is 1 s-orbital L = 1 corresponds to a p-orbital, the shape is dual-lobe along th ...
The Chemistry of Solutions Page | 1 Unit 7: The Chemistry of
... At what temperature do saturated solutions of sodium chloride and potassium chloride contain the same mass of solute per 100 mL of water? _____________ ...
... At what temperature do saturated solutions of sodium chloride and potassium chloride contain the same mass of solute per 100 mL of water? _____________ ...
Loeblein chemistry clicker questions2013
... •Describe a molecular model of gas pressure •Describe what happens to the measurable quantities if changes to the gas system are made. •Make sense of the measurable quantities of gases by analyzing examples of macroscopic things that are similar •Explain using physics what is happening on a molecula ...
... •Describe a molecular model of gas pressure •Describe what happens to the measurable quantities if changes to the gas system are made. •Make sense of the measurable quantities of gases by analyzing examples of macroscopic things that are similar •Explain using physics what is happening on a molecula ...
Role of Water as a Solvent
... hydroxide and potassium hydrogenphthalate (KHP) to standardize the base solution, by placing 50.00 mg of solid potassium hydrogenphthalate in a flask with a few drops of an indicator. A buret is filled with the base, and the initial buret reading is 0.55 ml; at the end of the titration the buret rea ...
... hydroxide and potassium hydrogenphthalate (KHP) to standardize the base solution, by placing 50.00 mg of solid potassium hydrogenphthalate in a flask with a few drops of an indicator. A buret is filled with the base, and the initial buret reading is 0.55 ml; at the end of the titration the buret rea ...
In chemistry, a salt is an ionic compound that
... ammonia) of the component ions. That slow, partial decomposition is usually accelerated by the presence of water, since hydrolysis is the other half of the reversible reaction equation of formation of weak salts. ...
... ammonia) of the component ions. That slow, partial decomposition is usually accelerated by the presence of water, since hydrolysis is the other half of the reversible reaction equation of formation of weak salts. ...
PowerPoint
... Oxidation-Reduction Reactions • “Redox Reactions” – Involve the transfer of one or more electrons from one substance to another – Examples • Formation of compounds from its elements and vice versa • Combustion reactions • Reactions that produce electricity in batteries • Cellular Respiration (energ ...
... Oxidation-Reduction Reactions • “Redox Reactions” – Involve the transfer of one or more electrons from one substance to another – Examples • Formation of compounds from its elements and vice versa • Combustion reactions • Reactions that produce electricity in batteries • Cellular Respiration (energ ...
Equilibrium
... Ksp Solubility Product Constant • Ksp is the equilibrium constant between an ionic solute and its ions in a saturated solution. • A very small Ksp indicates that only a small amount of solid will dissolve in water. • Ksp is equal to the product of the concentration of the ions in the equilibrium, e ...
... Ksp Solubility Product Constant • Ksp is the equilibrium constant between an ionic solute and its ions in a saturated solution. • A very small Ksp indicates that only a small amount of solid will dissolve in water. • Ksp is equal to the product of the concentration of the ions in the equilibrium, e ...
Second Semester Review Part 1
... (E) The solubility of most solids in water decreases with increasing temperature. 112. The solubility of a substance is 60 g per 100 mL water at 15 °C. A solution of the same substance is prepared by dissolving 75 g per 100 mL water at 75 °C and then is cooled slowly to 15 °C without any solid separ ...
... (E) The solubility of most solids in water decreases with increasing temperature. 112. The solubility of a substance is 60 g per 100 mL water at 15 °C. A solution of the same substance is prepared by dissolving 75 g per 100 mL water at 75 °C and then is cooled slowly to 15 °C without any solid separ ...
Solutions - iBioKaare
... The molar boiling point constant is the ratio of the elevation in boiling point to (a) molarity (b) molality (c) mole fraction of solvent (d) less than that of water An aqueous solution of methanol in water has vapour pressure (a) equal to that of water (b) equation to that of methanol (c) more than ...
... The molar boiling point constant is the ratio of the elevation in boiling point to (a) molarity (b) molality (c) mole fraction of solvent (d) less than that of water An aqueous solution of methanol in water has vapour pressure (a) equal to that of water (b) equation to that of methanol (c) more than ...
AP Chemistry Summer Assignment
... A and B as well as an introduction to the a few concepts in the first three chapters of the AP Chemistry Textbook that we haven’t covered yet. Having the following skills will be essential to your success in AP Chemistry and I will expect that you already have a firm grasp on these topics as we star ...
... A and B as well as an introduction to the a few concepts in the first three chapters of the AP Chemistry Textbook that we haven’t covered yet. Having the following skills will be essential to your success in AP Chemistry and I will expect that you already have a firm grasp on these topics as we star ...
Preface from the Textbook - McGraw Hill Higher Education
... students to first plan a logical approach, and only then proceed to the arithmetic solution. A check step, universally recommended by instructors, fosters the habit of considering the reasonableness and magnitude of the answer. For practice and reinforcement, each worked problem has a matched follow- ...
... students to first plan a logical approach, and only then proceed to the arithmetic solution. A check step, universally recommended by instructors, fosters the habit of considering the reasonableness and magnitude of the answer. For practice and reinforcement, each worked problem has a matched follow- ...
Chapter 4 Aqueous Reactions and Solution Stoichiometry
... • Solutions are homogeneous mixtures of two or more substances. • Solvent – the substance present in a solution in the greatest proportion (in number of moles). • Solute - the substance dissolved in the solvent. Aqueous Solution (aq) – a solution where water is the solvent. ...
... • Solutions are homogeneous mixtures of two or more substances. • Solvent – the substance present in a solution in the greatest proportion (in number of moles). • Solute - the substance dissolved in the solvent. Aqueous Solution (aq) – a solution where water is the solvent. ...
QA1
... Solutions of some cations give precipitates of sulphides (often highly coloured). Those of lead(II), silver(I), mercury(II), copper(II), iron(II), cobalt(II) and nickel(II) are back, bismuth(III) and tin(II) are brown, cadmium(II), tin(IV), and arsenic(III) and (V) are yellow, antimony(III) and (V) ...
... Solutions of some cations give precipitates of sulphides (often highly coloured). Those of lead(II), silver(I), mercury(II), copper(II), iron(II), cobalt(II) and nickel(II) are back, bismuth(III) and tin(II) are brown, cadmium(II), tin(IV), and arsenic(III) and (V) are yellow, antimony(III) and (V) ...
CHEM 30
... - theories of acids and bases: Arrhenius, Bronsted-Lowry, Lewis - applying all three theories of acids and bases: completing neutralization reactions, Bronsted-lowry - equations and recognizing conjugate acids and bases - predicting the pH of salt solutions - using the water constant, Kw – calculati ...
... - theories of acids and bases: Arrhenius, Bronsted-Lowry, Lewis - applying all three theories of acids and bases: completing neutralization reactions, Bronsted-lowry - equations and recognizing conjugate acids and bases - predicting the pH of salt solutions - using the water constant, Kw – calculati ...
Implicit Solvation Models - Center for Research Computing
... e. g., TIP3P uses δ+ = 0.417 and δ– = –0.834. These kinds of calculations are generally restricted to research groups that specialize in such things, and good “black box” implementations are not generally available. Efficient simplified models can implicitly treat the various physical influences of ...
... e. g., TIP3P uses δ+ = 0.417 and δ– = –0.834. These kinds of calculations are generally restricted to research groups that specialize in such things, and good “black box” implementations are not generally available. Efficient simplified models can implicitly treat the various physical influences of ...
File
... addition of the catalyst. Explain the role of the catalyst in changing the rate of the reaction. (c) If the temperature is increased, will the ratio kf/kr increase, remain the same, or decrease? Justify your answer with a one or two sentence explanation. [k f and kr are the specific rate constants f ...
... addition of the catalyst. Explain the role of the catalyst in changing the rate of the reaction. (c) If the temperature is increased, will the ratio kf/kr increase, remain the same, or decrease? Justify your answer with a one or two sentence explanation. [k f and kr are the specific rate constants f ...
Exam Review_Key_All Topics.082
... A Fractionating column is used to separate a mixture into its component parts by differences in boiling points. The fractionating column is filled with beads that allow for condensation. The bottom of the flask is heated and the gaseous vapours rise in the column. As vapours travel up the column the ...
... A Fractionating column is used to separate a mixture into its component parts by differences in boiling points. The fractionating column is filled with beads that allow for condensation. The bottom of the flask is heated and the gaseous vapours rise in the column. As vapours travel up the column the ...
Lab 6
... Action of CaCl2; When a neutral solution of a tartarate is treated with CaC12 solution a white ppt. of calcium tartarate is separated on the cold after scratching and stirring ...
... Action of CaCl2; When a neutral solution of a tartarate is treated with CaC12 solution a white ppt. of calcium tartarate is separated on the cold after scratching and stirring ...
Review #7: Solutions, Acids and Bases 1. Definitions: a) Solution: a
... i) % W / V: a description of the concentration of a solution. Tells the mass of solute per 100 mL of solution. For example, a 5% W/V solution of sugar would contain 5 g of sugar in 100 mL of solution. j) % W / W: a description of the concentration of a solution. Tells the mass of solute per 100 g of ...
... i) % W / V: a description of the concentration of a solution. Tells the mass of solute per 100 mL of solution. For example, a 5% W/V solution of sugar would contain 5 g of sugar in 100 mL of solution. j) % W / W: a description of the concentration of a solution. Tells the mass of solute per 100 g of ...
m5zn_1ed95c16cede0b1
... solute is able to dissolve. A saturated solution represents an equilibrium: – the rate of dissolving is equal to the rate of crystallization. The salt continues to dissolve, but crystallizes at the same rate so that there “appears” to be nothing happening. ...
... solute is able to dissolve. A saturated solution represents an equilibrium: – the rate of dissolving is equal to the rate of crystallization. The salt continues to dissolve, but crystallizes at the same rate so that there “appears” to be nothing happening. ...
CH 17 Study Guide with answer Key
... reactants and products, changing the volume of the reaction vessel causes no (11) ________________________ in the equilibrium. Changing the temperature of a reaction at equilibrium alters both the equilibrium (12) ________________________ and the equilibrium position. When a reaction is (13) _______ ...
... reactants and products, changing the volume of the reaction vessel causes no (11) ________________________ in the equilibrium. Changing the temperature of a reaction at equilibrium alters both the equilibrium (12) ________________________ and the equilibrium position. When a reaction is (13) _______ ...
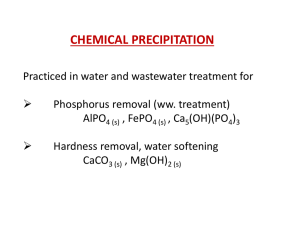
Phosphorus Removal from Wastewater by Chemical Precipitation
... The chemistry of phosphate precipitate formation is complex because of complexes formed between phosphate and metals, and between metals and other ligands in the wastewater. Side reactions of the metals with alkalinity to form hydroxide precipitates are another factor to be considered. The am ...
... The chemistry of phosphate precipitate formation is complex because of complexes formed between phosphate and metals, and between metals and other ligands in the wastewater. Side reactions of the metals with alkalinity to form hydroxide precipitates are another factor to be considered. The am ...