I- Introduction
... complete is called the end point, that is, when a change in some property of the solution is detected. The end point should coincide with the equivalence point. 6- The reaction should be quantitative. That is, the equilibrium of the reaction should be far to the right so that a sufficiently sharp ch ...
... complete is called the end point, that is, when a change in some property of the solution is detected. The end point should coincide with the equivalence point. 6- The reaction should be quantitative. That is, the equilibrium of the reaction should be far to the right so that a sufficiently sharp ch ...
IONIC EQULIBRIUM
... anions of all strong acid like Cl−, NO3−, ClO4− etc. are neutral anions. Same is true for cations of strong bases like K+, Na+, Ba++ etc. When they are dissolved in water, they do not react with water (i.e. they do not undergo hydrolysis) and these ions do not cause any change in pH of water (others ...
... anions of all strong acid like Cl−, NO3−, ClO4− etc. are neutral anions. Same is true for cations of strong bases like K+, Na+, Ba++ etc. When they are dissolved in water, they do not react with water (i.e. they do not undergo hydrolysis) and these ions do not cause any change in pH of water (others ...
Final Exam - Dawson College
... Toluene, C7H8 is a component of gasoline (octane, C8H18). It is present in gasoline as an octane booster at concentrations between 3 to 5% by mass (25% in racing cars gasoline). Consider a solution of octane with 20.% by mass of toluene at 20°C a. Calculate the total vapor pressure of this solution ...
... Toluene, C7H8 is a component of gasoline (octane, C8H18). It is present in gasoline as an octane booster at concentrations between 3 to 5% by mass (25% in racing cars gasoline). Consider a solution of octane with 20.% by mass of toluene at 20°C a. Calculate the total vapor pressure of this solution ...
Chapter 2 Geochemical Reactions
... ions in solution. While often classified as acid-base reactions, they are more than this, ranging from simple acid dissociation reactions to mineral dissolution, ion hydration and formation of complex ions. pH and dissociation of acids The most fundamental of all aqueous geochemical reactions is the ...
... ions in solution. While often classified as acid-base reactions, they are more than this, ranging from simple acid dissociation reactions to mineral dissolution, ion hydration and formation of complex ions. pH and dissociation of acids The most fundamental of all aqueous geochemical reactions is the ...
Answer
... • In the spaces provided, explain the meanings of the following terms. You may use an equation or diagram where appropriate. (a) hydrogen bonding An unusually strong dipole-dipole interaction that forms when a hydrogen atom is bonded to one of the very electronegative atoms F, O or N. ...
... • In the spaces provided, explain the meanings of the following terms. You may use an equation or diagram where appropriate. (a) hydrogen bonding An unusually strong dipole-dipole interaction that forms when a hydrogen atom is bonded to one of the very electronegative atoms F, O or N. ...
analytical chemistry - Львівський національний медичний
... The equilibrium-constant expression is defined in terms of the balanced chemical equation. All analytical reactions, as a rule, run in solutions. For solutions we can not change the pressure. Sometimes we might heat or freeze the reaction vessel. But, in general, all reactions (processes) occur at i ...
... The equilibrium-constant expression is defined in terms of the balanced chemical equation. All analytical reactions, as a rule, run in solutions. For solutions we can not change the pressure. Sometimes we might heat or freeze the reaction vessel. But, in general, all reactions (processes) occur at i ...
NH 4 1+
... Now let’s look at the second reason a double replacement reaction might occur: the formation of a weak acid. An acid is a compound that has an H+ ion bonded to some negative ion: HNO3 for example is nitric acid. HF is hydrofluoric acid. All acids fall into one of two categories: strong acids and wea ...
... Now let’s look at the second reason a double replacement reaction might occur: the formation of a weak acid. An acid is a compound that has an H+ ion bonded to some negative ion: HNO3 for example is nitric acid. HF is hydrofluoric acid. All acids fall into one of two categories: strong acids and wea ...
chemical change
... The evidence for a chemical reaction occurring, is the formation of a substance which is different from the original reactant or reactant, this is often accompanied by changes in energy, which are measured as temperature changes. Thus for the reaction of the silver metal sodium with the green/yellow ...
... The evidence for a chemical reaction occurring, is the formation of a substance which is different from the original reactant or reactant, this is often accompanied by changes in energy, which are measured as temperature changes. Thus for the reaction of the silver metal sodium with the green/yellow ...
Chapter 16 Aqueous Ionic Equilibrium Lecture Presentation
... • All ionic compounds dissolve in water to some degree. – However, many compounds have such low solubility in water that we classify them as insoluble. ...
... • All ionic compounds dissolve in water to some degree. – However, many compounds have such low solubility in water that we classify them as insoluble. ...
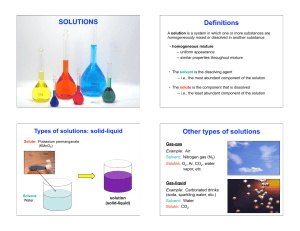
Chapter 4 Solution Chemistry
... aqueous solution, in which the solvent is water. • In this chapter, we’ll see how some types of chemical reactions take place and how we can organize chemical reactions into different types. Most of these reactions will take place in aqueous solutions. ...
... aqueous solution, in which the solvent is water. • In this chapter, we’ll see how some types of chemical reactions take place and how we can organize chemical reactions into different types. Most of these reactions will take place in aqueous solutions. ...
chapter4-bur.2917051..
... A base is a substance that forms OH- ion when added to water (Arrhenius definition). A strong soluble base is a soluble hydroxide compound that completely dissociates when added to water. An insoluble base is an insoluble hydroxide compound. There are also a few substances that act as weak bases in ...
... A base is a substance that forms OH- ion when added to water (Arrhenius definition). A strong soluble base is a soluble hydroxide compound that completely dissociates when added to water. An insoluble base is an insoluble hydroxide compound. There are also a few substances that act as weak bases in ...
Signs of Reaction - Calderglen High School
... The atoms of a compound are joined together. The substances in a mixture are NOT joined and can be easily separated. Calderglen High School ...
... The atoms of a compound are joined together. The substances in a mixture are NOT joined and can be easily separated. Calderglen High School ...
ppt
... examples of physical and chemical equilibrium systems. Explain the concept of chemical equilibrium and how it applies to the concentration of reactants and products in a chemical reaction at equilibrium. Create and complete an ICE table for an equilibrium system. Draw graphs of c vs. t to illustrate ...
... examples of physical and chemical equilibrium systems. Explain the concept of chemical equilibrium and how it applies to the concentration of reactants and products in a chemical reaction at equilibrium. Create and complete an ICE table for an equilibrium system. Draw graphs of c vs. t to illustrate ...
Common Student Misconceptions
... A substance with a solubility of less than 0.01 mol/L is regarded as being insoluble. Experimental observations have led to empirical guidelines for predicting the solubility. Solubility guidelines for common ionic compounds in water: • Compounds containing alkali metal ions or ammonium ion are solu ...
... A substance with a solubility of less than 0.01 mol/L is regarded as being insoluble. Experimental observations have led to empirical guidelines for predicting the solubility. Solubility guidelines for common ionic compounds in water: • Compounds containing alkali metal ions or ammonium ion are solu ...
acid
... A base is a substance that forms OH- ion when added to water (Arrhenius definition). A strong soluble base is a soluble hydroxide compound that completely dissociates when added to water. An insoluble base is an insoluble hydroxide compound. There are also a few substances that act as weak bases in ...
... A base is a substance that forms OH- ion when added to water (Arrhenius definition). A strong soluble base is a soluble hydroxide compound that completely dissociates when added to water. An insoluble base is an insoluble hydroxide compound. There are also a few substances that act as weak bases in ...
Last Name Professor BEAMER First Name
... Solution/Explanation: You are converting between particles (molecules) and mass (grams). Therefore, you need to use Avogadro’s Number. ...
... Solution/Explanation: You are converting between particles (molecules) and mass (grams). Therefore, you need to use Avogadro’s Number. ...
Lecture 25 Notes
... The hydronium ion A hydrogen ion (H+) does not exist by itself in an aqueous solution • In water, H+ combines with a polar H2O molecule to form a hydrated hydrogen ion (H3O+) called a hydronium ion ...
... The hydronium ion A hydrogen ion (H+) does not exist by itself in an aqueous solution • In water, H+ combines with a polar H2O molecule to form a hydrated hydrogen ion (H3O+) called a hydronium ion ...
Ch 17 Equilibrium
... to lower the pressure, because there are left fewer moles of gas on that side of the equation. ...
... to lower the pressure, because there are left fewer moles of gas on that side of the equation. ...
Introduction to Qualitative Analysis
... substances present in a given sample. We are not concerned with the quantity of each substance, but only whether certain substances are present or absent. On the other hand, in quantitative analysis we are concerned with determining the amount of each component present in a sample. For example, thos ...
... substances present in a given sample. We are not concerned with the quantity of each substance, but only whether certain substances are present or absent. On the other hand, in quantitative analysis we are concerned with determining the amount of each component present in a sample. For example, thos ...
高雄醫學大學九十二學年度學士後醫學系招生考試試題 科目:化學 考試
... (A) Both NaCl and CuS precipitate from solution. (B) No precipitate forms. (C) CuS will precipitate from solution (D) NaCl will precipitate from solution. (E) No reaction will occur. 65. Body temperature is about 308 K. On a cold day, what volume of air at 273 K must a person with a lung capacity of ...
... (A) Both NaCl and CuS precipitate from solution. (B) No precipitate forms. (C) CuS will precipitate from solution (D) NaCl will precipitate from solution. (E) No reaction will occur. 65. Body temperature is about 308 K. On a cold day, what volume of air at 273 K must a person with a lung capacity of ...
Solubility Workbook
... understanding of all aspects of the solubility unit. Ask yourself, “do I want to do well in this class?” If you are determined to be successful the minimum expectation that you should have for yourself is that you do all of these questions by the due dates given by your teacher. There are other thin ...
... understanding of all aspects of the solubility unit. Ask yourself, “do I want to do well in this class?” If you are determined to be successful the minimum expectation that you should have for yourself is that you do all of these questions by the due dates given by your teacher. There are other thin ...
Chapter 16 Aqueous Ionic Equilibrium Lecture Presentation
... • All ionic compounds dissolve in water to some degree. – However, many compounds have such low solubility in water that we classify them as insoluble. ...
... • All ionic compounds dissolve in water to some degree. – However, many compounds have such low solubility in water that we classify them as insoluble. ...
Experiment 22
... Although the product, [H+] [OH-] is small, that does not mean that both concentrations are necessarily small. If, for example, we dissolve HCl in water, the HCl in the solution will dissociate completely to H+ and Cl- ions; in 1 M HCl, [H+] will become 1 M, and there is nothing that Reaction 3 can ...
... Although the product, [H+] [OH-] is small, that does not mean that both concentrations are necessarily small. If, for example, we dissolve HCl in water, the HCl in the solution will dissociate completely to H+ and Cl- ions; in 1 M HCl, [H+] will become 1 M, and there is nothing that Reaction 3 can ...
UNITS OF CONCENTRATION
... takes into account the actual number of reacting species per mole of reagent (i.e., protons in the case of acid/base reactions or electrons in the case of redox reactions). For acids, an equivalent is defined as one mole of protons. The equivalent amount of any acid is the amount of acid that delive ...
... takes into account the actual number of reacting species per mole of reagent (i.e., protons in the case of acid/base reactions or electrons in the case of redox reactions). For acids, an equivalent is defined as one mole of protons. The equivalent amount of any acid is the amount of acid that delive ...