Chemistry lesson note
... APPLICATION OF CHEMISTRY • FOOD:- Chemistry is used to increase food production by the use of fertilizer and insecticides, preservation and addition of essential nutrients to improve the quality of food • CLOTHING:- Textile fibres are produced by chemical research • HOUSING:- Cement, concretes, bri ...
... APPLICATION OF CHEMISTRY • FOOD:- Chemistry is used to increase food production by the use of fertilizer and insecticides, preservation and addition of essential nutrients to improve the quality of food • CLOTHING:- Textile fibres are produced by chemical research • HOUSING:- Cement, concretes, bri ...
CHAPTER 6 Thermodynamics
... is constant” or sometimes expressed as "Energy can neither be created nor destroyed". Another approach is to say that the total energy of an isolated system remains constant. ...
... is constant” or sometimes expressed as "Energy can neither be created nor destroyed". Another approach is to say that the total energy of an isolated system remains constant. ...
Fundamentals of Equilibrium Thermodynamics
... energy difference only depends on the equilibrium states, not on the process. Here we have reached the first law of thermodynamics. The processes described under 3) are needed to define/measure the change in energy associated with the transformation A B . The states themselves are unique (from 1), ...
... energy difference only depends on the equilibrium states, not on the process. Here we have reached the first law of thermodynamics. The processes described under 3) are needed to define/measure the change in energy associated with the transformation A B . The states themselves are unique (from 1), ...
homework
... Students in this course need not compete with one another. Students may find cooperative studying advantageous. Since supplementary material, as well as textbook, may be included on the mid term and final examinations, students are urged to attend class faithfully. Please keep in mind that the goal ...
... Students in this course need not compete with one another. Students may find cooperative studying advantageous. Since supplementary material, as well as textbook, may be included on the mid term and final examinations, students are urged to attend class faithfully. Please keep in mind that the goal ...
Lecture 12
... Criteria for Spontaneity Criteria for spontaneity & equilibrium: (1) Is a reaction spontaneous? (2) When rxn reaches equilibrium, what is the ratio of products & reactants? A. H and U: not satisfactory Just because the energy of a system is lowered does not mean that this is automatically the direc ...
... Criteria for Spontaneity Criteria for spontaneity & equilibrium: (1) Is a reaction spontaneous? (2) When rxn reaches equilibrium, what is the ratio of products & reactants? A. H and U: not satisfactory Just because the energy of a system is lowered does not mean that this is automatically the direc ...
Chapter 2. The First Law
... • The means by which a system can exchange energy with its surroundings in terms of the work it may do or the heat that it may produce • The target concept is enthalpy, very useful for keeping track of the heat output (or requirements) of physical processes and chemical reactions at constant pressur ...
... • The means by which a system can exchange energy with its surroundings in terms of the work it may do or the heat that it may produce • The target concept is enthalpy, very useful for keeping track of the heat output (or requirements) of physical processes and chemical reactions at constant pressur ...
Equilibrium Constant
... react to give back the original reactants, even as the reactants are forming more products. After some time, both the forward and reverse reactions will be going on at the same rate. When this occurs, the reaction is said to have reached equilibrium. ...
... react to give back the original reactants, even as the reactants are forming more products. After some time, both the forward and reverse reactions will be going on at the same rate. When this occurs, the reaction is said to have reached equilibrium. ...
Modified Osteo-Odonto Keratoprosthesis (MOOKP) in patients
... Ben Lam, Srinivas Rao, Alvin Young, Lulu Cheng,Dennis Lam Hong Kong Eye Hospital Department of Ophthalmology and visual science /CUHK (no financial interest) ...
... Ben Lam, Srinivas Rao, Alvin Young, Lulu Cheng,Dennis Lam Hong Kong Eye Hospital Department of Ophthalmology and visual science /CUHK (no financial interest) ...
chemical reaction
... energy states • neither mass or energy can be _______ or _________ in a chemical reaction. • Energy can be • __________ from one object to ...
... energy states • neither mass or energy can be _______ or _________ in a chemical reaction. • Energy can be • __________ from one object to ...
chemical reaction
... • MORE energy is required to break the bonds in the reactants than is released by the formation of the ...
... • MORE energy is required to break the bonds in the reactants than is released by the formation of the ...
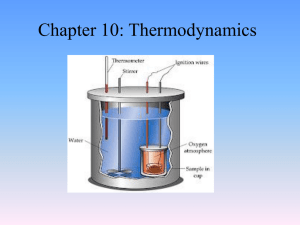
Chapter 10: Thermodynamics
... • A total of 135 J of work is done on a gaseous refrigerant as it undergoes compression. If the internal energy of the gas increases by 114 J during the process, what is the total amount of energy transferred as heat? ...
... • A total of 135 J of work is done on a gaseous refrigerant as it undergoes compression. If the internal energy of the gas increases by 114 J during the process, what is the total amount of energy transferred as heat? ...
Eighth Grade Review - PAMS-Doyle
... A chemical equation represents the change that takes place in a chemical reaction • In a chemical equation, the chemical formulas of the reactants are written on the left; an arrow indicates a change to a new substance; and the chemical formulas of the products are written on the right ...
... A chemical equation represents the change that takes place in a chemical reaction • In a chemical equation, the chemical formulas of the reactants are written on the left; an arrow indicates a change to a new substance; and the chemical formulas of the products are written on the right ...
Chemistry Comes Alive
... Makes sure that the substance always contains exactly same number of solute particles ...
... Makes sure that the substance always contains exactly same number of solute particles ...
Chemical Reactions
... • Spontaneous reactions—occur naturally, the process is unaided. • Example: –Decomposition of dead matter = spontaneous endothermic reactions. (absorbs heat energy) –Forest fire = spontaneous exothermic reactions. (releases heat energy) ...
... • Spontaneous reactions—occur naturally, the process is unaided. • Example: –Decomposition of dead matter = spontaneous endothermic reactions. (absorbs heat energy) –Forest fire = spontaneous exothermic reactions. (releases heat energy) ...
Faculty of Science Department of chemistry Practical Physical
... Course Description: The Experiments: We will perform at least 12 experiments in physical chemistry covering Thermodynamics and Equilibrium. Students in the laboratory course will rotate through the list of experiments in 2 groups, designated I and II to best utilize our available equipments. You wil ...
... Course Description: The Experiments: We will perform at least 12 experiments in physical chemistry covering Thermodynamics and Equilibrium. Students in the laboratory course will rotate through the list of experiments in 2 groups, designated I and II to best utilize our available equipments. You wil ...
Thermodynamics and kinetics
... • Change in external conditions can change equilibrium A stressed system at equilibrium will shift to reduce stress concentration, pressure, temperature • N2 + 3 H2 <--> 2 NH3 + 22 kcal What is the shift due to Increased temperature? Increased N2? Reduction of reactor vessel volume? ...
... • Change in external conditions can change equilibrium A stressed system at equilibrium will shift to reduce stress concentration, pressure, temperature • N2 + 3 H2 <--> 2 NH3 + 22 kcal What is the shift due to Increased temperature? Increased N2? Reduction of reactor vessel volume? ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.