Document
... Bi4Ti3O12(BTO) ceramic samples with different degrees of grain orientation (I) were prepared using the solid state reaction. Under the identical temperature, when the sintering time (2-20 h) increases, the I increases initially and reaches its maximum value of 89.9% ...
... Bi4Ti3O12(BTO) ceramic samples with different degrees of grain orientation (I) were prepared using the solid state reaction. Under the identical temperature, when the sintering time (2-20 h) increases, the I increases initially and reaches its maximum value of 89.9% ...
Chapter 15 Chemical Equilibrium
... 2. For those species for which both the initial and equilibrium concentrations are known, calculate the change in concentration that occurs as the system reaches equilibrium. 3. Use the stoichiometry of the reaction (that is, use the coefficients in the balanced chemical equation) to calculate the c ...
... 2. For those species for which both the initial and equilibrium concentrations are known, calculate the change in concentration that occurs as the system reaches equilibrium. 3. Use the stoichiometry of the reaction (that is, use the coefficients in the balanced chemical equation) to calculate the c ...
Fundamental Equilibrium Concepts
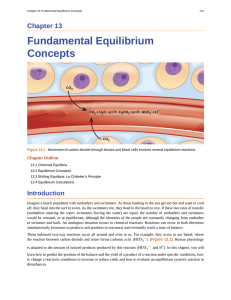
... Imagine a beach populated with sunbathers and swimmers. As those basking in the sun get too hot and want to cool off, they head into the surf to swim. As the swimmers tire, they head to the beach to rest. If these two rates of transfer (sunbathers entering the water, swimmers leaving the water) are ...
... Imagine a beach populated with sunbathers and swimmers. As those basking in the sun get too hot and want to cool off, they head into the surf to swim. As the swimmers tire, they head to the beach to rest. If these two rates of transfer (sunbathers entering the water, swimmers leaving the water) are ...
Document
... is 0.113 at 298 K, which corresponds to a standard free-energy change of 5.4 kJ/mol. In a certain experiment, the initial pressures are PN2O4 = 0.453 atm and PNO2 = 0.122 atm. Calculate ΔG for the reaction at these pressures, and predict the direction in which the reaction will proceed spontaneously ...
... is 0.113 at 298 K, which corresponds to a standard free-energy change of 5.4 kJ/mol. In a certain experiment, the initial pressures are PN2O4 = 0.453 atm and PNO2 = 0.122 atm. Calculate ΔG for the reaction at these pressures, and predict the direction in which the reaction will proceed spontaneously ...
Overview of Reference Electrodes and Alternative
... For an electrode reaction to occur within an electrochemical cell, there must be at least two electrodes; the working electrode facilitates electron transfer to the analyte of interest while the counter electrode maintains electroneutrality by participating in a reaction of opposite sign. Though it ...
... For an electrode reaction to occur within an electrochemical cell, there must be at least two electrodes; the working electrode facilitates electron transfer to the analyte of interest while the counter electrode maintains electroneutrality by participating in a reaction of opposite sign. Though it ...
sample
... B) Physical changes alter the composition of the substances involved. C) Physical properties are not valid characteristics for identifying a substance. D) Physical properties are mostly extensive in nature. E) Physical changes are usually accompanied by chemical changes. Ans: A Difficulty: E 3. Sele ...
... B) Physical changes alter the composition of the substances involved. C) Physical properties are not valid characteristics for identifying a substance. D) Physical properties are mostly extensive in nature. E) Physical changes are usually accompanied by chemical changes. Ans: A Difficulty: E 3. Sele ...
FREE Sample Here
... B) Physical changes alter the composition of the substances involved. C) Physical properties are not valid characteristics for identifying a substance. D) Physical properties are mostly extensive in nature. E) Physical changes are usually accompanied by chemical changes. Ans: A Difficulty: E 3. Sele ...
... B) Physical changes alter the composition of the substances involved. C) Physical properties are not valid characteristics for identifying a substance. D) Physical properties are mostly extensive in nature. E) Physical changes are usually accompanied by chemical changes. Ans: A Difficulty: E 3. Sele ...
102MSJc14 - Louisiana Tech University
... Any chemical reaction could be considered as a forward and backward reactions occurring at the same time( ) as described previously. If the rates of backward and forward reactions chemical reactions are comparable both reactants and products can coexist leading to a condition called chemical equilib ...
... Any chemical reaction could be considered as a forward and backward reactions occurring at the same time( ) as described previously. If the rates of backward and forward reactions chemical reactions are comparable both reactants and products can coexist leading to a condition called chemical equilib ...