An Introduction to Matter
... decomposed by a chemical change into simpler substances – An element is a pure substance which cannot be broken down into anything simpler by either physical or chemical means. ...
... decomposed by a chemical change into simpler substances – An element is a pure substance which cannot be broken down into anything simpler by either physical or chemical means. ...
Class 10 - Department of Physics | Oregon State
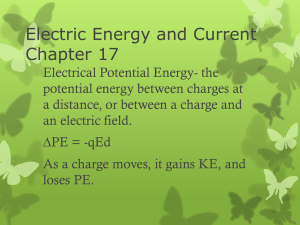
... In the study and harnessing of electrical energy, it is convenient to express the electrical potential energy on a per-unit-charge basis. This is called the electric potential or voltage, and is denoted by V. Electric potential is a field—a point-by-point description of space— but it’s an energy fie ...
... In the study and harnessing of electrical energy, it is convenient to express the electrical potential energy on a per-unit-charge basis. This is called the electric potential or voltage, and is denoted by V. Electric potential is a field—a point-by-point description of space— but it’s an energy fie ...
Estimating Mineral Weathering Rates in Catskills
... ◘ Basic Cations: Ca, Mg, K, Na ◘ Silica: H4SiO4 ◘ Aluminum: potentially toxic to aquatic biota ...
... ◘ Basic Cations: Ca, Mg, K, Na ◘ Silica: H4SiO4 ◘ Aluminum: potentially toxic to aquatic biota ...
PHYS 241 Recitation
... – Potential is not the same as potential energy, but they are intimately related – Electrostatic potential energy is not the same as potential energy of a particle. The former is the work to construct the entire configuration, while the later is the work required to bring that one particle in from i ...
... – Potential is not the same as potential energy, but they are intimately related – Electrostatic potential energy is not the same as potential energy of a particle. The former is the work to construct the entire configuration, while the later is the work required to bring that one particle in from i ...
Chemical Changes(Lab report about Nacl,Cuso4,Cacl2 mixed)
... 1.Stick 3 notes on the test tubes 2.Add 5 ml cuso4 and 5ml Cacl2 to the A 3.Shaked them and watch what happened 4.Add 5 ml Cacl2 and 5ml Nacl to the B 5.Shaked,Watch what happened 6.Add 5ml Nacl and 5ml cuso4 to the C 7.Shaked,Watch what happened 8.Record 9.Put the equipments back to the table 10.ga ...
... 1.Stick 3 notes on the test tubes 2.Add 5 ml cuso4 and 5ml Cacl2 to the A 3.Shaked them and watch what happened 4.Add 5 ml Cacl2 and 5ml Nacl to the B 5.Shaked,Watch what happened 6.Add 5ml Nacl and 5ml cuso4 to the C 7.Shaked,Watch what happened 8.Record 9.Put the equipments back to the table 10.ga ...
Electrostatics Notes 4 – Electric Potential, Electric Potential
... energy - that is it has the potential to move in that field. Note that the potential energy it has could be used to… A non-uniform field, such as that provided by a point, is one which has a different… ...
... energy - that is it has the potential to move in that field. Note that the potential energy it has could be used to… A non-uniform field, such as that provided by a point, is one which has a different… ...
Reading-Chem v Phys
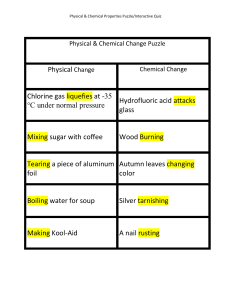
... … Several occurrences are common indicators of a chemical change. Energy is usually absorbed or evolved in chemical reactions. Burning coal evolves heat; cooking food absorbs heat. Energy is also absorbed or evolved, however, in physical changes of state. A color change, as in leaves turning in the ...
... … Several occurrences are common indicators of a chemical change. Energy is usually absorbed or evolved in chemical reactions. Burning coal evolves heat; cooking food absorbs heat. Energy is also absorbed or evolved, however, in physical changes of state. A color change, as in leaves turning in the ...
Chapter 4
... 3. If 85.6 mL of a 6.75 M solution is diluted to 6.20 L with water, what is the concentration of the final solution? 4. 24.0 g of ethane (C2H6) are burned to form CO2 and H2O. How many grams of CO2 are ...
... 3. If 85.6 mL of a 6.75 M solution is diluted to 6.20 L with water, what is the concentration of the final solution? 4. 24.0 g of ethane (C2H6) are burned to form CO2 and H2O. How many grams of CO2 are ...
Chapter 2 Reading Guide
... Make an electron distribution diagram of water. Which element is most electronegative? Why is water considered a polar molecule? Label the regions that are more positive or more negative. (This is a very important concept. Spend some time with this one!) ...
... Make an electron distribution diagram of water. Which element is most electronegative? Why is water considered a polar molecule? Label the regions that are more positive or more negative. (This is a very important concept. Spend some time with this one!) ...
Physical and Chemical Properties Worksheet Multiple Choice
... 5. ( Physical or Chemical ) properties are not as easy to observe. ...
... 5. ( Physical or Chemical ) properties are not as easy to observe. ...
Chapter 15 - cloudfront.net
... multiple elements • Salt is a combination of sodium and chloride (NaCl) ...
... multiple elements • Salt is a combination of sodium and chloride (NaCl) ...
durfee high school science department
... 9/15 M All 3 sections hand back and review element & equipment quiz. Begin States of Matter and KMT. Group work sheet on phase change graph. Issue texts Hand lab on physical and chemical properties and changes discuss pre-lab questions ticket to entry ...
... 9/15 M All 3 sections hand back and review element & equipment quiz. Begin States of Matter and KMT. Group work sheet on phase change graph. Issue texts Hand lab on physical and chemical properties and changes discuss pre-lab questions ticket to entry ...