Unit 10 Worksheet 5
... 2. Calculate the gravitational potential difference between: a) point A and the base of the hill b) point B and the base of the hill c) point C and point A d) point C and point E e) Would an object gain more energy going from D to A or from E to F? Why? ...
... 2. Calculate the gravitational potential difference between: a) point A and the base of the hill b) point B and the base of the hill c) point C and point A d) point C and point E e) Would an object gain more energy going from D to A or from E to F? Why? ...
Matter and Its Changes
... Elements: Cannot be broken down into any other substance by chemical or physical means ...
... Elements: Cannot be broken down into any other substance by chemical or physical means ...
Slide 1
... substances ability to participate in chemical reactions. (Change into new matter.) –Flammability •ability to burn •Burning wood creates ash and smoke ...
... substances ability to participate in chemical reactions. (Change into new matter.) –Flammability •ability to burn •Burning wood creates ash and smoke ...
Chapter One Outline
... Identifying Matter: Physical Properties Physical properties can be observed and measured without changing the composition of a substance. Examples include temperature, mass, density, etc. Density is the ratio of an objects mass to its volume; D = m/v Chemical Properties A substances chemical propert ...
... Identifying Matter: Physical Properties Physical properties can be observed and measured without changing the composition of a substance. Examples include temperature, mass, density, etc. Density is the ratio of an objects mass to its volume; D = m/v Chemical Properties A substances chemical propert ...
Physics 30 Concept Check 6 Concept: Calculate the electric
... Determine the amount of potential difference between two parallel plates that are separated by 6.0 cm if the electric field between the plates is determined to be ...
... Determine the amount of potential difference between two parallel plates that are separated by 6.0 cm if the electric field between the plates is determined to be ...
Key - Sardis Secondary
... 15. What is the relationship between the kinetic energy of molecules and their physical state? ...
... 15. What is the relationship between the kinetic energy of molecules and their physical state? ...
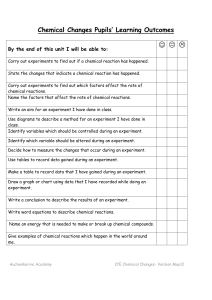
Chemistry Standards Checklist
... SC6. Students will understand the effects motion of atoms and molecules in chemical and physical processes. a. Compare and contrast atomic/molecular motion in solids, liquids, gases, and plasmas. ...
... SC6. Students will understand the effects motion of atoms and molecules in chemical and physical processes. a. Compare and contrast atomic/molecular motion in solids, liquids, gases, and plasmas. ...
Introduction to Chemistry
... Ionic- Two elements bond by transferring electrons to create ions that attract together (+ is attracted to - after an electron is transferred) ...
... Ionic- Two elements bond by transferring electrons to create ions that attract together (+ is attracted to - after an electron is transferred) ...
TERM 2 Unit 3 YR 9 SCI It is elementary
... understandings of atomic structure. Students model an atom according to currently accepted understandings. They will identify patterns in atomic structure that allow prediction of the products of chemical reactions and are reflected by the periodic table. They recognise that new substances are forme ...
... understandings of atomic structure. Students model an atom according to currently accepted understandings. They will identify patterns in atomic structure that allow prediction of the products of chemical reactions and are reflected by the periodic table. They recognise that new substances are forme ...