Describing Matter Chapter 2:2 Physical and Chemical Properties
... describes a substance based on its ability to change into a new substance with different properties • Chemical Change~ a change that occurs when one or more substances are changed into entirely new substances with different properties; cannot be reversed using physical means • Density~ the amount of ...
... describes a substance based on its ability to change into a new substance with different properties • Chemical Change~ a change that occurs when one or more substances are changed into entirely new substances with different properties; cannot be reversed using physical means • Density~ the amount of ...
17.1 Electric Potential and Potential Difference
... will move it towards the negative plate. The work done on the positive charge is f x d, and this work results in the particle gaining kinetic energy (f x d). Thus it must lose potential energy equal to -(f x d). The electric field was previously defined as the (electric) force per unit charge. Elect ...
... will move it towards the negative plate. The work done on the positive charge is f x d, and this work results in the particle gaining kinetic energy (f x d). Thus it must lose potential energy equal to -(f x d). The electric field was previously defined as the (electric) force per unit charge. Elect ...
PHYS_2326_020309
... done by external force required to move charges into the certain geometry (closer or farther apart). ...
... done by external force required to move charges into the certain geometry (closer or farther apart). ...
The chemical master equation
... condition is met within a molecule (e.g. IVR putting enough energy in a reactive mode). In a well-mixed system, the collisions necessary for a reaction to occur are random events. ...
... condition is met within a molecule (e.g. IVR putting enough energy in a reactive mode). In a well-mixed system, the collisions necessary for a reaction to occur are random events. ...
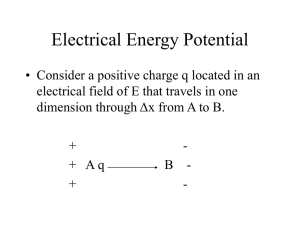
Electrical Energy Potential
... Capacitance • A capacitor is a device that temporarily stores electrical energy that can be reclaimed at a later time. It consists of two parallel metal plates separated by a distance d, each connected to one of the terminals of an electrical source. The plate connected to the +ve terminal losses e ...
... Capacitance • A capacitor is a device that temporarily stores electrical energy that can be reclaimed at a later time. It consists of two parallel metal plates separated by a distance d, each connected to one of the terminals of an electrical source. The plate connected to the +ve terminal losses e ...
Electric Fields and Potential
... different parallel plates are hooked up to different voltages one becomes more negative and the other one becomes more positive. This difference is what causes the charge to be stored. A capacitor is discharged when there is a conducting path between the two conducting plates. This is when people ca ...
... different parallel plates are hooked up to different voltages one becomes more negative and the other one becomes more positive. This difference is what causes the charge to be stored. A capacitor is discharged when there is a conducting path between the two conducting plates. This is when people ca ...
PowerPoint
... and the isobaric water-gas shift reaction proceeds to equilibrium, what is the CO conversion if the temperature is (a) 150 °C, (b) 250 °C and (c) 350 °C? ‣ Noting that the water-gas shift reaction is exothermic; predict whether the equilibrium conversion will increase or decrease as the temperature ...
... and the isobaric water-gas shift reaction proceeds to equilibrium, what is the CO conversion if the temperature is (a) 150 °C, (b) 250 °C and (c) 350 °C? ‣ Noting that the water-gas shift reaction is exothermic; predict whether the equilibrium conversion will increase or decrease as the temperature ...