Lecture Notes V
... Heat of Formation Heat of reaction of fuels combusting in air or oxygen with starting and ending points at 25 OC and 1 atm gives fuel heating value. These values are tabulated for common fuels. This cannot be applied to all reactions - needs enormous amounts of tabulated data. Instead selected react ...
... Heat of Formation Heat of reaction of fuels combusting in air or oxygen with starting and ending points at 25 OC and 1 atm gives fuel heating value. These values are tabulated for common fuels. This cannot be applied to all reactions - needs enormous amounts of tabulated data. Instead selected react ...
CHEM 313 - Suraj @ LUMS
... There will be class quizzes and homework assignments on week-to-week basis. There will be additional class assignments along with a mid-term exam and final exam. Class quizzes will be taken as and when the instructor wishes to do so. Each homework assignment will involve class-based exercises and ke ...
... There will be class quizzes and homework assignments on week-to-week basis. There will be additional class assignments along with a mid-term exam and final exam. Class quizzes will be taken as and when the instructor wishes to do so. Each homework assignment will involve class-based exercises and ke ...
Utah - Wavefunction, Inc.
... matter how they are rearranged; the total mass stays the same. Although energy can be absorbed or released in a chemical reaction, the total amount of energy and matter in it remains constant. Many reactions attain a state of equilibrium. Many ordinary activities, such as baking, involve chemical re ...
... matter how they are rearranged; the total mass stays the same. Although energy can be absorbed or released in a chemical reaction, the total amount of energy and matter in it remains constant. Many reactions attain a state of equilibrium. Many ordinary activities, such as baking, involve chemical re ...
The Periodic table and subatomic particles
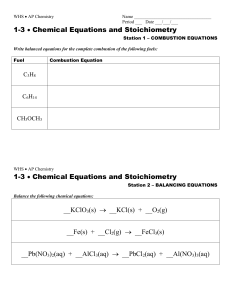
... ____ Mn + ____ HI ____ H2 + ____ MnI3 7. Why do we need to balance chemical equations? 8. A 10th grade science class is studying the law of conservation of mass. Reactants, magnesium and hydrochloric acid are massed before and after their reaction in an open Erlenmeyer flask. The following data is ...
... ____ Mn + ____ HI ____ H2 + ____ MnI3 7. Why do we need to balance chemical equations? 8. A 10th grade science class is studying the law of conservation of mass. Reactants, magnesium and hydrochloric acid are massed before and after their reaction in an open Erlenmeyer flask. The following data is ...
CE3503 Expectations – Equilibrium Reactions that proceed to
... Reactions that proceed to equilibrium slowly (hours, e.g. BOD exertion, to decades, radioisotope decay) require a kinetic approach as introduced during our discussion of kinetics, reactors and mass balance. Those that proceed more rapidly (milliseconds, e.g. dissociation of strong acid, to minutes, ...
... Reactions that proceed to equilibrium slowly (hours, e.g. BOD exertion, to decades, radioisotope decay) require a kinetic approach as introduced during our discussion of kinetics, reactors and mass balance. Those that proceed more rapidly (milliseconds, e.g. dissociation of strong acid, to minutes, ...
Ch. 3 9-Station Review
... A student is assigned the task of determining the number of moles of water in one mole of MgCl2 · n H2O. The student collects the data shown in the following table. Mass of empty container Initial mass of sample and container Mass of sample and container after first heating Mass of sample and contai ...
... A student is assigned the task of determining the number of moles of water in one mole of MgCl2 · n H2O. The student collects the data shown in the following table. Mass of empty container Initial mass of sample and container Mass of sample and container after first heating Mass of sample and contai ...
Matter Anything that has mass and takes up space (volume).
... Physical Change With a physical change no new substance is created and the original matter can be recovered. Physical change does not change the composition of the matter. The original matter is still present. The substance may seem different, but the way the atoms are linked up are the same. ...
... Physical Change With a physical change no new substance is created and the original matter can be recovered. Physical change does not change the composition of the matter. The original matter is still present. The substance may seem different, but the way the atoms are linked up are the same. ...
PHY481 - Lecture 8: Energy in a charge distribution, capacitance
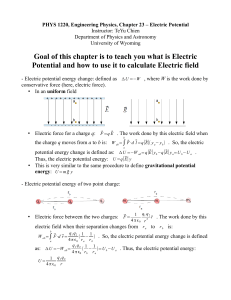
... Potential energy stored in a charge distribution A different question is: What is the potential energy of a distribution of charges, that is, what is the potential energy stored in a distribution of charges. In this case we muts take into account the way in which the electrostatic potential changes ...
... Potential energy stored in a charge distribution A different question is: What is the potential energy of a distribution of charges, that is, what is the potential energy stored in a distribution of charges. In this case we muts take into account the way in which the electrostatic potential changes ...
Slide 1
... the accompanying text in their classes. All recipients of this work are expected to abide by these restrictions and to honor the intended pedagogical purposes and the needs of other instructors who rely on these materials. ...
... the accompanying text in their classes. All recipients of this work are expected to abide by these restrictions and to honor the intended pedagogical purposes and the needs of other instructors who rely on these materials. ...
Why Study Chemistry
... – how hot or cold something is (a physical property) – related to the average (kinetic) energy of the substance (not the total energy) – Measured in units of • Degrees Fahrenheit (oF) • Degrees Celsius (oC) • Kelvin (K) ...
... – how hot or cold something is (a physical property) – related to the average (kinetic) energy of the substance (not the total energy) – Measured in units of • Degrees Fahrenheit (oF) • Degrees Celsius (oC) • Kelvin (K) ...