Unit 12 - HKU Physics
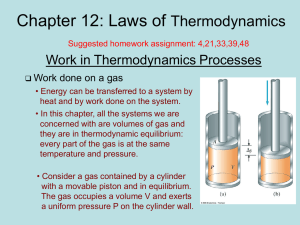
... Consider the following reversible processes at constant temperature, the piston moves down without friction. Applying the first law of thermodynamics, ∆U = Q + W , and making use the property of ideal gas which has its internal energy depends only on the temperature, we have ∆U = 0 and Q = −W , sinc ...
... Consider the following reversible processes at constant temperature, the piston moves down without friction. Applying the first law of thermodynamics, ∆U = Q + W , and making use the property of ideal gas which has its internal energy depends only on the temperature, we have ∆U = 0 and Q = −W , sinc ...
time: - 90 minutes
... 180 m/s. If the inlet area of the nozzle is 80 cm2 , determine the mass flow rate and the exit area of the nozzle. (2) A frictionless piston cylinder device initially contains 200L of saturated refrigerant134a. The piston is free to move and its mass is such that it maintains a pressure of 800kPa on ...
... 180 m/s. If the inlet area of the nozzle is 80 cm2 , determine the mass flow rate and the exit area of the nozzle. (2) A frictionless piston cylinder device initially contains 200L of saturated refrigerant134a. The piston is free to move and its mass is such that it maintains a pressure of 800kPa on ...
fridge in space
... energy is 1keV (the XRS is designed to detect x-rays of energy 0.4-12keV). If the detector absorbs too many photons, there is a possibility that its temperature might go up substantially. In order to prevent this from happening, the XRS will be attached to a fridge unit to keep it "cool." To attain ...
... energy is 1keV (the XRS is designed to detect x-rays of energy 0.4-12keV). If the detector absorbs too many photons, there is a possibility that its temperature might go up substantially. In order to prevent this from happening, the XRS will be attached to a fridge unit to keep it "cool." To attain ...
Historical burdens on physics 112 Thermal energy
... physical quantity in the usual sense of the word. One says that it is a “process quantity” since it makes no sense to ask for its values for a given system in a given state. Chemists prefer to manage with another “surrogate” quantity, the enthalpy. This quantity behaves like a heat content, but only ...
... physical quantity in the usual sense of the word. One says that it is a “process quantity” since it makes no sense to ask for its values for a given system in a given state. Chemists prefer to manage with another “surrogate” quantity, the enthalpy. This quantity behaves like a heat content, but only ...
Energy and Heat Transfer
... Energy cannot be created or destroyed Energy lost during one process must equal the energy gained during another Heat can spontaneously flow from a hotter object to a cooler object, but not the other way around ...
... Energy cannot be created or destroyed Energy lost during one process must equal the energy gained during another Heat can spontaneously flow from a hotter object to a cooler object, but not the other way around ...
Lecture12
... during expansion. Estimate the work done by the gas on the piston during this adiabatic expansion by assuming the engine cylinder contains 0.100 moles of an ideal monatomic gas which goes from 1.20x103 K to 4.00x102 K typical engine temperatures, during the ...
... during expansion. Estimate the work done by the gas on the piston during this adiabatic expansion by assuming the engine cylinder contains 0.100 moles of an ideal monatomic gas which goes from 1.20x103 K to 4.00x102 K typical engine temperatures, during the ...
Classical thermodynamics of particles in harmonic traps
... these processes involve reversible work; reversible work can be effected only by external forces acting on the walls to change the volume. For a harmonically confined gas, processes such as stirring can increase the spatial extent of the gas without a change in the spring constant, but reversible wo ...
... these processes involve reversible work; reversible work can be effected only by external forces acting on the walls to change the volume. For a harmonically confined gas, processes such as stirring can increase the spatial extent of the gas without a change in the spring constant, but reversible wo ...
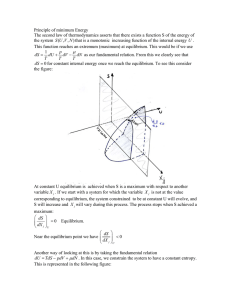
Principle of minimum Energy The second law of thermodynamics
... forced trough a porous plug or obstruction in the pipe. The cylinder and the pistons are A volume V1 gas is forces though at an initial pressure and temperature Pi , Ti . The cylinder and the pistons are insulating. Upon passing through the porous membrane, real gases usually cool down. So T f < Ti ...
... forced trough a porous plug or obstruction in the pipe. The cylinder and the pistons are A volume V1 gas is forces though at an initial pressure and temperature Pi , Ti . The cylinder and the pistons are insulating. Upon passing through the porous membrane, real gases usually cool down. So T f < Ti ...
Energy - ability of an object to do work
... Kinetic energy – the actual movement of an object Mechanical – when potential and kinetic energy are put to an object Electric energy – form of moving energy that has a flow of electric charges Circuit- a path in which electrons move Conductor – materials that allow electrons, sound, or heat to move ...
... Kinetic energy – the actual movement of an object Mechanical – when potential and kinetic energy are put to an object Electric energy – form of moving energy that has a flow of electric charges Circuit- a path in which electrons move Conductor – materials that allow electrons, sound, or heat to move ...
Notes
... Note that heat capacity applies to objects, while molar and specific heat capacities apply to substances. Note also that the units of each can specify degrees Celsius or Kelvin, since the magnitude of a degree is the same on both scales. The device used to measure heat flow between systems and surro ...
... Note that heat capacity applies to objects, while molar and specific heat capacities apply to substances. Note also that the units of each can specify degrees Celsius or Kelvin, since the magnitude of a degree is the same on both scales. The device used to measure heat flow between systems and surro ...