Chemical Thermodynamics
... The difference between E and H is the amount of expansion work (P V work) that the system can do. Unless there is a change in the number of moles of gas present, this difference is extremely small and can usually be neglected. For an ideal gas, PV = nRT. At constant temperature and pressure, P ...
... The difference between E and H is the amount of expansion work (P V work) that the system can do. Unless there is a change in the number of moles of gas present, this difference is extremely small and can usually be neglected. For an ideal gas, PV = nRT. At constant temperature and pressure, P ...
File - SRIT - MECHANICAL ENGINEERING
... ECV is the change in energy content of the control volume in t seconds. QCV is the heat energy entered into the control volume in t seconds. WCV is the work energy left the control volume in t seconds. hi & h0 are specific enthalpy of the inlet and outlet streams respectively. ...
... ECV is the change in energy content of the control volume in t seconds. QCV is the heat energy entered into the control volume in t seconds. WCV is the work energy left the control volume in t seconds. hi & h0 are specific enthalpy of the inlet and outlet streams respectively. ...
Chapter 2. The First Law
... 1. Heating is the transfer of energy that makes use of disorderly molecular motion (thermal motion) in the surroundings 2. Work is the transfer of energy that makes use of organized motion in the surrounding 3. The distinction between work and heat is made in the surroundings ...
... 1. Heating is the transfer of energy that makes use of disorderly molecular motion (thermal motion) in the surroundings 2. Work is the transfer of energy that makes use of organized motion in the surrounding 3. The distinction between work and heat is made in the surroundings ...
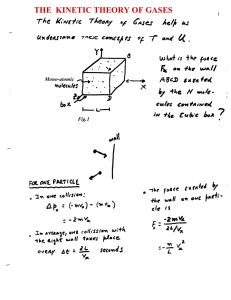
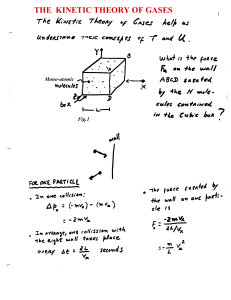
The kinetic theory of the gases
... As an application of the expression above for the equation of state, let’s consider an adiabatic process, one in which the gas container is thermally well isolated, i.e. no heat-transfer occurs. No heat-transfer is added or removed to the gas, Q = 0 As the piston is pressed, work is done on the gas ...
... As an application of the expression above for the equation of state, let’s consider an adiabatic process, one in which the gas container is thermally well isolated, i.e. no heat-transfer occurs. No heat-transfer is added or removed to the gas, Q = 0 As the piston is pressed, work is done on the gas ...
thermodynamics
... 24. The process in the system the variables (PTV) changes vary slowly so that the system remain in thermal and mechanical equilibrium with its surrounding throughout is called quasi-static process. 25. A process in which the temperature of the system is kept constant throughout is called isothermal ...
... 24. The process in the system the variables (PTV) changes vary slowly so that the system remain in thermal and mechanical equilibrium with its surrounding throughout is called quasi-static process. 25. A process in which the temperature of the system is kept constant throughout is called isothermal ...
heat engine
... temperature, and all the rejected heat goes into a cold reservoir at a single temperature. Since the efficiency can only depend on the reservoir temperatures, the ratio of heats can only depend on those temperatures. QC QH ...
... temperature, and all the rejected heat goes into a cold reservoir at a single temperature. Since the efficiency can only depend on the reservoir temperatures, the ratio of heats can only depend on those temperatures. QC QH ...
4.1 The Concepts of Force and Mass
... temperature, and all the rejected heat goes into a cold reservoir at a single temperature. Since the efficiency can only depend on the reservoir temperatures, the ratio of heats can only depend on those temperatures. QC QH ...
... temperature, and all the rejected heat goes into a cold reservoir at a single temperature. Since the efficiency can only depend on the reservoir temperatures, the ratio of heats can only depend on those temperatures. QC QH ...
Example
... A calorimeter contains 1kg of water, assume there is no heat loss,a piece of 500g food rise the temperature of water 3oC, how many energy does the food contain each gram? ...
... A calorimeter contains 1kg of water, assume there is no heat loss,a piece of 500g food rise the temperature of water 3oC, how many energy does the food contain each gram? ...
Chapter 15
... or isovolumetric. If we want the gas to return to its original state without changing temperature, we must trace a curve from point C to A along an isotherm. Note that an isotherm on a PV diagram is not a straight line. The work done during the process ABCA is the area enclosed by the graph, since W ...
... or isovolumetric. If we want the gas to return to its original state without changing temperature, we must trace a curve from point C to A along an isotherm. Note that an isotherm on a PV diagram is not a straight line. The work done during the process ABCA is the area enclosed by the graph, since W ...
File
... force acts on it to change its motion. 2nd Law: F=ma – force = mass x acceleration. If you throw a ball harder (more force), it will accelerate faster and go farther. 3rd Law: Action – Reaction. One object exerts a force on a 2nd object (action), and the 2nd exerts an equal and opposite force back o ...
... force acts on it to change its motion. 2nd Law: F=ma – force = mass x acceleration. If you throw a ball harder (more force), it will accelerate faster and go farther. 3rd Law: Action – Reaction. One object exerts a force on a 2nd object (action), and the 2nd exerts an equal and opposite force back o ...
Chapter 15
... or isovolumetric. If we want the gas to return to its original state without changing temperature, we must trace a curve from point C to A along an isotherm. Note that an isotherm on a PV diagram is not a straight line. The work done during the process ABCA is the area enclosed by the graph, since W ...
... or isovolumetric. If we want the gas to return to its original state without changing temperature, we must trace a curve from point C to A along an isotherm. Note that an isotherm on a PV diagram is not a straight line. The work done during the process ABCA is the area enclosed by the graph, since W ...
Effect of temperature dependent specific heats
... Internal energy, kJ Piston speed, m/s Cylinder volume, m3 Clearance volume, m3 Displacement volume, m3 Distance from top dead center, m Burning rate of the fuel, dimensionless Average cylinder gas velocity, m/s Angle, degree Start of combustion or heat addition, degree Duration of combustion, degree ...
... Internal energy, kJ Piston speed, m/s Cylinder volume, m3 Clearance volume, m3 Displacement volume, m3 Distance from top dead center, m Burning rate of the fuel, dimensionless Average cylinder gas velocity, m/s Angle, degree Start of combustion or heat addition, degree Duration of combustion, degree ...
Solutions to TI4: First Law of Thermodynamics
... 1. Consider the human body as a system and apply the first law of thermodynamics to it.. a. Internal energy is related to temperature. The human body has fairly constant temperature, hence the internal energy does not decrease as described above. b. Internal energy is added to the body to balance th ...
... 1. Consider the human body as a system and apply the first law of thermodynamics to it.. a. Internal energy is related to temperature. The human body has fairly constant temperature, hence the internal energy does not decrease as described above. b. Internal energy is added to the body to balance th ...
Thermodynamics - Faculty
... 2. The Carnot cycle (see Figure 12.17 in your textbook) can be described in 4 steps: a) Step 1: The cycle starts with the piston positioned such that V is at a minimum. At this point, heat Q is added to the system through a heat reservoir at a high temperature TH . The system absorbs the heat a cons ...
... 2. The Carnot cycle (see Figure 12.17 in your textbook) can be described in 4 steps: a) Step 1: The cycle starts with the piston positioned such that V is at a minimum. At this point, heat Q is added to the system through a heat reservoir at a high temperature TH . The system absorbs the heat a cons ...
PHYS-2010: General Physics I Course Lecture Notes Section XIV Dr. Donald G. Luttermoser
... 2. The Carnot cycle (see Figure 12.17 in your textbook) can be described in 4 steps: a) Step 1: The cycle starts with the piston positioned such that V is at a minimum. At this point, heat Q is added to the system through a heat reservoir at a high temperature TH . The system absorbs the heat a cons ...
... 2. The Carnot cycle (see Figure 12.17 in your textbook) can be described in 4 steps: a) Step 1: The cycle starts with the piston positioned such that V is at a minimum. At this point, heat Q is added to the system through a heat reservoir at a high temperature TH . The system absorbs the heat a cons ...