Lecture notes Chapters 10
... Potential energy (PE): is stored energy. The potential energy possessed by an object arises from its capacity to move or to cause motion. Chemical energy and nuclear energy are examples of potential energy. Law of conservation of energy: energy can be neither created nor destroyed but one form of en ...
... Potential energy (PE): is stored energy. The potential energy possessed by an object arises from its capacity to move or to cause motion. Chemical energy and nuclear energy are examples of potential energy. Law of conservation of energy: energy can be neither created nor destroyed but one form of en ...
physical chemistry lecture 3
... •Some examples include energy (and many other thermodynamic terms), pressure, volume, altitude, distance, etc. • An energy change in a system can occur by many different combinations of heat (q) and work (w), but no matter what the combination, ΔU is always the same — the amount of the energy change ...
... •Some examples include energy (and many other thermodynamic terms), pressure, volume, altitude, distance, etc. • An energy change in a system can occur by many different combinations of heat (q) and work (w), but no matter what the combination, ΔU is always the same — the amount of the energy change ...
Work Done by an Expanding Gas
... Various Gas Expansions: pV Plots and Work An ideal monatomic gas is contained in a cylinder with a movable piston so that the gas can do work on the outside world, and heat can be added or removed as necessary. The ...
... Various Gas Expansions: pV Plots and Work An ideal monatomic gas is contained in a cylinder with a movable piston so that the gas can do work on the outside world, and heat can be added or removed as necessary. The ...
ABL, Thermodynamics, Reynolds decomposition, Eddy covariance
... The potential temperature (θ) of a parcel of air at pressure P is the temperature that the parcel would acquire if adiabatically brought to a standard reference pressure P0 (= 1000 millibars). ...
... The potential temperature (θ) of a parcel of air at pressure P is the temperature that the parcel would acquire if adiabatically brought to a standard reference pressure P0 (= 1000 millibars). ...
Introductory Physics, High School
... 2.2 Interpret and provide examples of how energy can be converted from gravitational potential energy to kinetic energy and vice versa. 2.3 Describe both qualitatively and quantitatively how work can be expressed as a change in mechanical energy. 2.4 Describe both qualitatively and quantitatively th ...
... 2.2 Interpret and provide examples of how energy can be converted from gravitational potential energy to kinetic energy and vice versa. 2.3 Describe both qualitatively and quantitatively how work can be expressed as a change in mechanical energy. 2.4 Describe both qualitatively and quantitatively th ...
W - Boulder School for Condensed Matter and Materials Physics
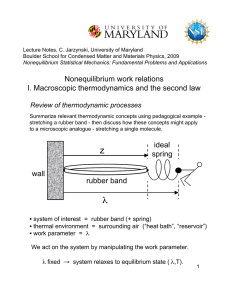
... in which a system of interest evolves from one equilibrium state to another. - focus on thermodynamic processes in which we act on the system by manipulating a work parameter λ The first and second laws of thermodynamics make predictions regarding such processes … ...
... in which a system of interest evolves from one equilibrium state to another. - focus on thermodynamic processes in which we act on the system by manipulating a work parameter λ The first and second laws of thermodynamics make predictions regarding such processes … ...
Energy, work and Power
... Gas Laws ( Question) Diesel fuel is injected into a cylinder at an inlet pressure of 1.2 bar and a temperature of 24oC. The compression ratio of the engine is 18 : 1 and the minimum ignition temperature of diesel fuel is 400oC. Calculate the minimum cylinder pressure in bar required to achieve this ...
... Gas Laws ( Question) Diesel fuel is injected into a cylinder at an inlet pressure of 1.2 bar and a temperature of 24oC. The compression ratio of the engine is 18 : 1 and the minimum ignition temperature of diesel fuel is 400oC. Calculate the minimum cylinder pressure in bar required to achieve this ...
Examples Paper 2 (1-2)
... will always tend to increase due to irreversibilities. If the system is not isolated (Q can flow) and is undergoing a reversible process, the differential of entropy is a minimum and can be defined as dS = dQ/T. In macroscopic terms, the second law is closely related to the reversibility of a proces ...
... will always tend to increase due to irreversibilities. If the system is not isolated (Q can flow) and is undergoing a reversible process, the differential of entropy is a minimum and can be defined as dS = dQ/T. In macroscopic terms, the second law is closely related to the reversibility of a proces ...
Isentropic Efficiency in Engineering Thermodynamics Introduction
... where dmCV /dt is the time rate of change of mass in the CV, and ṁi and ṁe are the inlet and outlet mass flow rates, all at time t. If there is more than one inlet and outlet, summation signs are added to the right-hand terms. ...
... where dmCV /dt is the time rate of change of mass in the CV, and ṁi and ṁe are the inlet and outlet mass flow rates, all at time t. If there is more than one inlet and outlet, summation signs are added to the right-hand terms. ...
Laws of Thermodynamics
... force that we consider. Then an infinitesimal change in the volume, dV , implies an infinitesimal amount of work done on the system, dW = −P dV . Note the sign: a positive change dV implies that the system expands a little, and hence that the system does work against the external pressure force (P i ...
... force that we consider. Then an infinitesimal change in the volume, dV , implies an infinitesimal amount of work done on the system, dW = −P dV . Note the sign: a positive change dV implies that the system expands a little, and hence that the system does work against the external pressure force (P i ...
SPH 4C - mackenziekim
... What is work? What is energy? What is the formula for calculating work? What are the units for work and energy? ...
... What is work? What is energy? What is the formula for calculating work? What are the units for work and energy? ...
Turbomachinery
... – Production of s must be positive – Every natural system, if left undisturbed, will change spontaneously and approach a state of equilibrium or rest. The property associated with the capability of systems for change is called entropy. ...
... – Production of s must be positive – Every natural system, if left undisturbed, will change spontaneously and approach a state of equilibrium or rest. The property associated with the capability of systems for change is called entropy. ...
Mechanical Engineering (Electrical Branch)
... Extensive properties per unit mass is specific extensive properties It is an intensive properties. For example; Specific volume, Specific energy ...
... Extensive properties per unit mass is specific extensive properties It is an intensive properties. For example; Specific volume, Specific energy ...
heat-and-temperature-are-not-same-thing
... Celsius. Note that the unit of temperature is written as °C, (not °c or ...
... Celsius. Note that the unit of temperature is written as °C, (not °c or ...
11 Thermodynamics and Thermochemistry
... constant pressure. When a reaction is allowed to take place in an open container a quantity of heat proportional to the quantity of matter present, will be released or absorbed. This flow of heat is the enthalpy change, ∆H. The units for ∆H are kJ (or kJ/mol). «Reactions that release heat are termed ...
... constant pressure. When a reaction is allowed to take place in an open container a quantity of heat proportional to the quantity of matter present, will be released or absorbed. This flow of heat is the enthalpy change, ∆H. The units for ∆H are kJ (or kJ/mol). «Reactions that release heat are termed ...
Heat Energy - Waconia High School
... Objects with potential energy are not doing work. They are storing energy in order to do work. ...
... Objects with potential energy are not doing work. They are storing energy in order to do work. ...