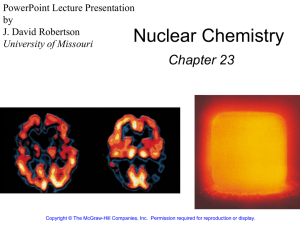
Chapter 1
... • Calculate n, the number of half-lives elapsed using the half-life as the conversion factor n = 2.0 days x 1 half-life / 0.5 days = 4 half lives • Calculate the amount remaining 10.0 ng 5.0 ng 2.5 ng 1.3 ng 0.63 ng 1st half-life ...
... • Calculate n, the number of half-lives elapsed using the half-life as the conversion factor n = 2.0 days x 1 half-life / 0.5 days = 4 half lives • Calculate the amount remaining 10.0 ng 5.0 ng 2.5 ng 1.3 ng 0.63 ng 1st half-life ...
nuclear force
... • A positron is emitted from the nucleus as a proton is converted into a neutron. • The atomic number decreases by one but the mass number stays the same. ...
... • A positron is emitted from the nucleus as a proton is converted into a neutron. • The atomic number decreases by one but the mass number stays the same. ...
Radioactivity
... 2. A high voltage is applied between the gauze and the wire. The voltage is adjusted until it is just below the value required to produce sparks. 3. When a radioactive source is brought near the wire gauze, the radiation ionizes the air below it. The motion of the ions to the gauze and the wire caus ...
... 2. A high voltage is applied between the gauze and the wire. The voltage is adjusted until it is just below the value required to produce sparks. 3. When a radioactive source is brought near the wire gauze, the radiation ionizes the air below it. The motion of the ions to the gauze and the wire caus ...
The Strong Nuclear Force and the Stability of the Nucleus
... ● Fusion means joining up two small nuclei to form a bigger nucleus. ● When two small nuclei the product of fusion would have more BE per nucleon. ● The increases in binding energy per nucleon are much larger for fusion than for fission reactions, because the graph increases more steeply for light n ...
... ● Fusion means joining up two small nuclei to form a bigger nucleus. ● When two small nuclei the product of fusion would have more BE per nucleon. ● The increases in binding energy per nucleon are much larger for fusion than for fission reactions, because the graph increases more steeply for light n ...
nuclear physics - review
... ● Fusion means joining up two small nuclei to form a bigger nucleus. ● When two small nuclei the product of fusion would have more BE per nucleon. ● The increases in binding energy per nucleon are much larger for fusion than for fission reactions, because the graph increases more steeply for light n ...
... ● Fusion means joining up two small nuclei to form a bigger nucleus. ● When two small nuclei the product of fusion would have more BE per nucleon. ● The increases in binding energy per nucleon are much larger for fusion than for fission reactions, because the graph increases more steeply for light n ...
Understanding Nuclear Power
... First let us take a look at Uranium, chemical element symbol “U”, which is used to fuel nuclear reactors. The atomic number of U is 92 which is the number of protons in the nucleus. The atomic mass number is the number of protons and neutrons in the nucleus. A chemical element can have atoms with di ...
... First let us take a look at Uranium, chemical element symbol “U”, which is used to fuel nuclear reactors. The atomic number of U is 92 which is the number of protons in the nucleus. The atomic mass number is the number of protons and neutrons in the nucleus. A chemical element can have atoms with di ...
Unit 3 Study Guide: Atomic Structure and Nuclear
... In a nuclear power plant, energy is produced in the reactor core by fission reactions that occur inuraniumcontaining bars called (18) ____________________. The uranium is found at location (19)_________________ in the diagram. The rate at which the nuclear reaction takes place is controlled by other ...
... In a nuclear power plant, energy is produced in the reactor core by fission reactions that occur inuraniumcontaining bars called (18) ____________________. The uranium is found at location (19)_________________ in the diagram. The rate at which the nuclear reaction takes place is controlled by other ...
File
... naturally in the environment – Examples – radioisotopes in air, water, rocks, plants and ...
... naturally in the environment – Examples – radioisotopes in air, water, rocks, plants and ...
Problem Set 7 Solutions
... shielded by dense, highZ materials. Therefore, lead and concrete are the best here: lead for its highZ and inexpensiveness, concrete for its high density and great inexpensiveness. Xrays are more easily shielded than gamma rays, but proceed according to the same mechanisms: all three photon effec ...
... shielded by dense, highZ materials. Therefore, lead and concrete are the best here: lead for its highZ and inexpensiveness, concrete for its high density and great inexpensiveness. Xrays are more easily shielded than gamma rays, but proceed according to the same mechanisms: all three photon effec ...
Nuc Chem PP - Liberty Union High School District
... A sample of 3x107 Radon atoms are trapped in a basement that is sealed. The half-life of Radon is 3.83 days. How many radon atoms are left after 31 days? answer:1.2x105 atoms ...
... A sample of 3x107 Radon atoms are trapped in a basement that is sealed. The half-life of Radon is 3.83 days. How many radon atoms are left after 31 days? answer:1.2x105 atoms ...
NAME GRADED: LET IT BEGIN!!! ____ / 30 pts DIRECTIONS: Use
... Necessary Background: When an isotope is a nuclear radioactive isotope, it means that it can spontaneously breakdown, by emitting alpha particles (effectively He-4 nuclei each equaling 2 protons and 2 neutrons, and of course, 0 electrons), beta particles (high speed e- from degenerating neutrons) or ...
... Necessary Background: When an isotope is a nuclear radioactive isotope, it means that it can spontaneously breakdown, by emitting alpha particles (effectively He-4 nuclei each equaling 2 protons and 2 neutrons, and of course, 0 electrons), beta particles (high speed e- from degenerating neutrons) or ...
Radiation and Radioactive Decay
... nuclear force, thus the binding energy increases. As we pass iron, we again have even more nucleons, however the distance between them is increasing and the mutual attraction due to the (short-range) strong nuclear force is weakened. But the proton pairs are still feeling the electromagnet repulsion ...
... nuclear force, thus the binding energy increases. As we pass iron, we again have even more nucleons, however the distance between them is increasing and the mutual attraction due to the (short-range) strong nuclear force is weakened. But the proton pairs are still feeling the electromagnet repulsion ...
Nuclear Chemistry Radioactivity
... – When uranium-235 undergoes fission, more neutrons are released creating the possibility of a chain reaction. – A chain reaction is a self-sustaining series of nuclear fissions caused by the absorption of neutrons released from previous nuclear fissions. ...
... – When uranium-235 undergoes fission, more neutrons are released creating the possibility of a chain reaction. – A chain reaction is a self-sustaining series of nuclear fissions caused by the absorption of neutrons released from previous nuclear fissions. ...
mass numbers
... A chain reaction occurs when a critical mass of uranium undergoes fission, releasing a large amount of heat and energy that produces an atomic explosion. ...
... A chain reaction occurs when a critical mass of uranium undergoes fission, releasing a large amount of heat and energy that produces an atomic explosion. ...
Chapter 4 The Liquid Drop Model
... whereas for nuclei with different numbers of protons and neutrons (for fixed A) the binding energy decreases as the square of the number difference. The spacing between energy levels is inversely proportional to the volume of the nucleus - this can be seen by treating the nucleus as a three-dimensio ...
... whereas for nuclei with different numbers of protons and neutrons (for fixed A) the binding energy decreases as the square of the number difference. The spacing between energy levels is inversely proportional to the volume of the nucleus - this can be seen by treating the nucleus as a three-dimensio ...
3 Background radiation
... Radioactive tracers are used to investigate a patient's body without the need for surgery. Gamma emitters and sometimes beta emitters are used. This is because gamma rays and beta particles can pass through skin, whereas alpha particles cannot. A chemical reaction or other process in which the produ ...
... Radioactive tracers are used to investigate a patient's body without the need for surgery. Gamma emitters and sometimes beta emitters are used. This is because gamma rays and beta particles can pass through skin, whereas alpha particles cannot. A chemical reaction or other process in which the produ ...
Atomic and Nuclear Terms
... When a gamma photon is absorbed the whole photon is absorbed so one photon can ionize only one atom. However, the emitted electron has so much energy that it can ionize further atoms leading to damage similar to that caused by alpha and beta. ...
... When a gamma photon is absorbed the whole photon is absorbed so one photon can ionize only one atom. However, the emitted electron has so much energy that it can ionize further atoms leading to damage similar to that caused by alpha and beta. ...
Chapter 29
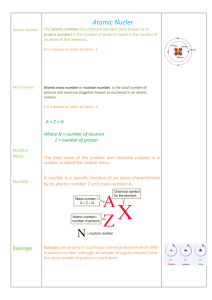
... • The atomic number, Z, equals the number of protons in the nucleus • The neutron number, N, is the number of neutrons in the nucleus • The mass number (not the same as the mass), A, is the number of nucleons in the nucleus: A = Z + N ...
... • The atomic number, Z, equals the number of protons in the nucleus • The neutron number, N, is the number of neutrons in the nucleus • The mass number (not the same as the mass), A, is the number of nucleons in the nucleus: A = Z + N ...
Atomic and Nuclear Terms
... When a gamma photon is absorbed the whole photon is absorbed so one photon can ionize only one atom. However, the emitted electron has so much energy that it can ionize further atoms leading to damage similar to that caused by alpha and beta. ...
... When a gamma photon is absorbed the whole photon is absorbed so one photon can ionize only one atom. However, the emitted electron has so much energy that it can ionize further atoms leading to damage similar to that caused by alpha and beta. ...
Chapter 29
... between protons • These forces should cause the nucleus to fly apart • The nuclei are stable because of the presence of another, short-range force, called the nuclear force • This is an attractive force that acts between all nuclear particles • The nuclear attractive force is stronger than the Coulo ...
... between protons • These forces should cause the nucleus to fly apart • The nuclei are stable because of the presence of another, short-range force, called the nuclear force • This is an attractive force that acts between all nuclear particles • The nuclear attractive force is stronger than the Coulo ...
12_physics_notes_ch13_nuclei
... The number of protons in the nucleus is called the atomic number. It is denoted by Z. • Mass number: The total number of protons and neutrons present in a nucleus is called the mass number of the element. It is denoted by A. • No. of Protons, Electrons, nucleons and Neutrons in an Atom: a) Number of ...
... The number of protons in the nucleus is called the atomic number. It is denoted by Z. • Mass number: The total number of protons and neutrons present in a nucleus is called the mass number of the element. It is denoted by A. • No. of Protons, Electrons, nucleons and Neutrons in an Atom: a) Number of ...
File
... Nuclear binding energy is the energy required to disassemble a nucleus into free unbound neutrons and protons. Nuclear binding energy can be calculated from the difference of mass of a nucleus, and the sum of the masses of the number of free neutrons and protons that make up the nucleus. This mass d ...
... Nuclear binding energy is the energy required to disassemble a nucleus into free unbound neutrons and protons. Nuclear binding energy can be calculated from the difference of mass of a nucleus, and the sum of the masses of the number of free neutrons and protons that make up the nucleus. This mass d ...
Atomic Nuclei - RAJEEV Classes
... Two nuclides are isotones if they have the very same neutron number N, but different proton number Z. For example, boron-12 and carbon-13 nuclei both contain 7 neutrons, and so are isotones . ...
... Two nuclides are isotones if they have the very same neutron number N, but different proton number Z. For example, boron-12 and carbon-13 nuclei both contain 7 neutrons, and so are isotones . ...
Radioactivityunit6
... • This is the process that drives our sun, and all other suns. • We can do it under the right conditions in a lab, but we end up putting in more energy than we get out. • The left over products of fusion are relatively safe, which is why a lot of research is going into developing fusion reactors. ...
... • This is the process that drives our sun, and all other suns. • We can do it under the right conditions in a lab, but we end up putting in more energy than we get out. • The left over products of fusion are relatively safe, which is why a lot of research is going into developing fusion reactors. ...
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is either a nuclear reaction or a radioactive decay process in which the nucleus of an atom splits into smaller parts (lighter nuclei). The fission process often produces free neutrons and photons (in the form of gamma rays), and releases a very large amount of energy even by the energetic standards of radioactive decay.Nuclear fission of heavy elements was discovered on December 17, 1938 by German Otto Hahn and his assistant Fritz Strassmann, and explained theoretically in January 1939 by Lise Meitner and her nephew Otto Robert Frisch. Frisch named the process by analogy with biological fission of living cells. It is an exothermic reaction which can release large amounts of energy both as electromagnetic radiation and as kinetic energy of the fragments (heating the bulk material where fission takes place). In order for fission to produce energy, the total binding energy of the resulting elements must be less negative (higher energy) than that of the starting element.Fission is a form of nuclear transmutation because the resulting fragments are not the same element as the original atom. The two nuclei produced are most often of comparable but slightly different sizes, typically with a mass ratio of products of about 3 to 2, for common fissile isotopes. Most fissions are binary fissions (producing two charged fragments), but occasionally (2 to 4 times per 1000 events), three positively charged fragments are produced, in a ternary fission. The smallest of these fragments in ternary processes ranges in size from a proton to an argon nucleus.Apart from fission induced by a neutron, harnessed and exploited by humans, a natural form of spontaneous radioactive decay (not requiring a neutron) is also referred to as fission, and occurs especially in very high-mass-number isotopes. Spontaneous fission was discovered in 1940 by Flyorov, Petrzhak and Kurchatov in Moscow, when they decided to confirm that, without bombardment by neutrons, the fission rate of uranium was indeed negligible, as predicted by Niels Bohr; it wasn't.The unpredictable composition of the products (which vary in a broad probabilistic and somewhat chaotic manner) distinguishes fission from purely quantum-tunnelling processes such as proton emission, alpha decay and cluster decay, which give the same products each time. Nuclear fission produces energy for nuclear power and drives the explosion of nuclear weapons. Both uses are possible because certain substances called nuclear fuels undergo fission when struck by fission neutrons, and in turn emit neutrons when they break apart. This makes possible a self-sustaining nuclear chain reaction that releases energy at a controlled rate in a nuclear reactor or at a very rapid uncontrolled rate in a nuclear weapon.The amount of free energy contained in nuclear fuel is millions of times the amount of free energy contained in a similar mass of chemical fuel such as gasoline, making nuclear fission a very dense source of energy. The products of nuclear fission, however, are on average far more radioactive than the heavy elements which are normally fissioned as fuel, and remain so for significant amounts of time, giving rise to a nuclear waste problem. Concerns over nuclear waste accumulation and over the destructive potential of nuclear weapons may counterbalance the desirable qualities of fission as an energy source, and give rise to ongoing political debate over nuclear power.