Lecture 16: Iron Core Collapse, Neutron Stars, and Nucleosynthesis
... (the positive charge of the protons is also important, but not dominant). Nuclei with Z = N are more tightly bound. The nuclear range is short range. The nucleons on one side of a large nucleus do not feel attracted by nucleons on the other side, only to their neighbors As the mass of the nucleus in ...
... (the positive charge of the protons is also important, but not dominant). Nuclei with Z = N are more tightly bound. The nuclear range is short range. The nucleons on one side of a large nucleus do not feel attracted by nucleons on the other side, only to their neighbors As the mass of the nucleus in ...
Chapter 28 for Chem
... remains the same. It changes into a DIFFERENT element (in this case from H to He). ...
... remains the same. It changes into a DIFFERENT element (in this case from H to He). ...
File
... Because of the existence of so many isotopes, the term element is sometimes confusing. The term nuclide is better. A nuclide is an atom that has a definite mass number A and Z-number. A list of ...
... Because of the existence of so many isotopes, the term element is sometimes confusing. The term nuclide is better. A nuclide is an atom that has a definite mass number A and Z-number. A list of ...
Nuclear Chemistry - Duplin County Schools
... • Because the atom now has one more proton, it becomes the element with an atomic number one greater than that of the original element. • However, because the total number of protons and neutrons does not change during beta decay, the mass number of the new element is the same as that of the origina ...
... • Because the atom now has one more proton, it becomes the element with an atomic number one greater than that of the original element. • However, because the total number of protons and neutrons does not change during beta decay, the mass number of the new element is the same as that of the origina ...
Balancing a Nuclear Equation
... Nuclear Fission • An isotope absorbs a neutron, becomes unstable, and then fissions by breaking into a couple of pieces and releasing one or more neutrons plus a large amount of energy • Nuclear fission is generally considered intentional ...
... Nuclear Fission • An isotope absorbs a neutron, becomes unstable, and then fissions by breaking into a couple of pieces and releasing one or more neutrons plus a large amount of energy • Nuclear fission is generally considered intentional ...
(or radioactive isotopes).
... • Gamma rays are used to kill bacteria, mould and insects in food. They are also used to kill bacteria on hospital equipment, dressings and bandages. • This is useful particularly on packaged food or on plastic items which would be damaged by heat sterilisation. • There are arguments for using cobal ...
... • Gamma rays are used to kill bacteria, mould and insects in food. They are also used to kill bacteria on hospital equipment, dressings and bandages. • This is useful particularly on packaged food or on plastic items which would be damaged by heat sterilisation. • There are arguments for using cobal ...
Nuclear Chemistry - Moorpark College
... (1:1 for lighter atoms increasing to 1:1.5 for heavier atoms). All nuclei with 84 or more protons are radioactive. Nuclear Force-Why don't the protons in the same nucleus repel? Attraction between nucleons acting at short distance (1 x 10-15 m) compensates for repulsion of electric charges. This att ...
... (1:1 for lighter atoms increasing to 1:1.5 for heavier atoms). All nuclei with 84 or more protons are radioactive. Nuclear Force-Why don't the protons in the same nucleus repel? Attraction between nucleons acting at short distance (1 x 10-15 m) compensates for repulsion of electric charges. This att ...
CHAPTER 4: ABUNDANCE AND RADIOACTIVITY OF UNSTABLE
... distribution is such that the maximum in this distribution is at an energy about one third of the maximum energy. As mentioned above, in some cases the resulting daughter nucleus is not in an excited state, so that no g radiation is emitted. This happens to be the case with the two isotopes that are ...
... distribution is such that the maximum in this distribution is at an energy about one third of the maximum energy. As mentioned above, in some cases the resulting daughter nucleus is not in an excited state, so that no g radiation is emitted. This happens to be the case with the two isotopes that are ...
Chapter 9 Nuclear Radiation 9.1 Natural Radioactivity Radioactive
... • an unstable nucleus of 236U undergoes fission (splits). • smaller nuclei are produced, such as Kr-91 and Ba-142. • neutrons are released to bombard more 235U. 1n ...
... • an unstable nucleus of 236U undergoes fission (splits). • smaller nuclei are produced, such as Kr-91 and Ba-142. • neutrons are released to bombard more 235U. 1n ...
Ch9
... In nuclear power plants, • fission is used to produce energy. • control rods in the reactor absorb neutrons to slow and control the chain reactions of fission. ...
... In nuclear power plants, • fission is used to produce energy. • control rods in the reactor absorb neutrons to slow and control the chain reactions of fission. ...
Physics 228 Today: April 22, 2012 Ch. 43 Nuclear
... In determining nuclear masses, we usually deal with atoms, so tabulated masses are usually for atoms. The atomic electron masses and binding energy make a small difference, but usually if we ignore the inclusion of electrons it is not an issue, since the number of nucleons and electrons is constant ...
... In determining nuclear masses, we usually deal with atoms, so tabulated masses are usually for atoms. The atomic electron masses and binding energy make a small difference, but usually if we ignore the inclusion of electrons it is not an issue, since the number of nucleons and electrons is constant ...
Photo chapter opener 21 Subatomic particle tracks in a bubble
... • A nuclide is a type of atom characterized by its proton number, neutron number and its energy condition. • Nuclides with identical proton number but differing neutron number are called isotopes. • Conditions with a life of less than 10-10s are called excited conditions of a nuclide. • At present, ...
... • A nuclide is a type of atom characterized by its proton number, neutron number and its energy condition. • Nuclides with identical proton number but differing neutron number are called isotopes. • Conditions with a life of less than 10-10s are called excited conditions of a nuclide. • At present, ...
Nuclear - PEO Scarborough Chapter
... neutrons. The electrons form a cloud around the nucleus. The neutrons hold mass containing particles (protons) together in the nucleus. When an atom has too much energy, it dissipates energy through emission of alpha (α), beta (β) and gamma (γ) radiation. This phenomenon is called radioactivity or r ...
... neutrons. The electrons form a cloud around the nucleus. The neutrons hold mass containing particles (protons) together in the nucleus. When an atom has too much energy, it dissipates energy through emission of alpha (α), beta (β) and gamma (γ) radiation. This phenomenon is called radioactivity or r ...
Nuclear Physics and Bombs
... To sustain the chain reaction, it is necessary to have many fissionable atoms around to catch neutrons. The smallest amount of fissile material is called critical mass. The critical mass depends on the density of the material and how easy for 235U to capture a neutron. Inside a nuclear power plant, ...
... To sustain the chain reaction, it is necessary to have many fissionable atoms around to catch neutrons. The smallest amount of fissile material is called critical mass. The critical mass depends on the density of the material and how easy for 235U to capture a neutron. Inside a nuclear power plant, ...
atomic number.
... in a fusion bomb or reactor can you overcome the Coulomb repulsion and force nucleons to fuse. ...
... in a fusion bomb or reactor can you overcome the Coulomb repulsion and force nucleons to fuse. ...
Word - The Physics Teacher.ie
... number of neutrons that are present. Control rods made of a neutron absorber capture neutrons. Absorbing more neutrons in a control rod means that there are fewer neutrons available to cause fission. Therefore, pushing the control rods deeper into the reactor will reduce its power output, and extrac ...
... number of neutrons that are present. Control rods made of a neutron absorber capture neutrons. Absorbing more neutrons in a control rod means that there are fewer neutrons available to cause fission. Therefore, pushing the control rods deeper into the reactor will reduce its power output, and extrac ...
The Band of Stability
... Radioactivity is the spontaneous emission of radiation by nuclei. Radioactive decay changes the nature and identity of an atom’s nucleus. This occurs for a specific reason. Elements from hydrogen to lead (atomic numbers 1-82) have stable isotopes in which the tendency of protons to repel one another ...
... Radioactivity is the spontaneous emission of radiation by nuclei. Radioactive decay changes the nature and identity of an atom’s nucleus. This occurs for a specific reason. Elements from hydrogen to lead (atomic numbers 1-82) have stable isotopes in which the tendency of protons to repel one another ...
Nuclear Physics and Radioactivity2
... released. 2. Be able to convert nuclear binding energy for one reaction to energy in Joules. Mass measured in electron volts Since mass and energy are related, 1 u = 1.6605 x 10-27 kg = 931.5 MeV. We can also look at a nucleus in terms of the forces that hold it together. The electric force describe ...
... released. 2. Be able to convert nuclear binding energy for one reaction to energy in Joules. Mass measured in electron volts Since mass and energy are related, 1 u = 1.6605 x 10-27 kg = 931.5 MeV. We can also look at a nucleus in terms of the forces that hold it together. The electric force describe ...
document
... Usually involve atoms with large nucleii such as the Lathanides and Actinides They produce , and emissions. Involve a nucleus collapsing to form a smaller nucleus ...
... Usually involve atoms with large nucleii such as the Lathanides and Actinides They produce , and emissions. Involve a nucleus collapsing to form a smaller nucleus ...
21J 2011 The Polywell Nuclear Reactor Website July 4, 2011
... overwhelmed by some type of highly attractive force. And since one atom's nucleus does not seem to be attracted to another's, it was thought that nuclear forces must be very strong and very limited in range. But if the nuclear force was so strong, how could atoms decay? In 1928 while studying in Ger ...
... overwhelmed by some type of highly attractive force. And since one atom's nucleus does not seem to be attracted to another's, it was thought that nuclear forces must be very strong and very limited in range. But if the nuclear force was so strong, how could atoms decay? In 1928 while studying in Ger ...
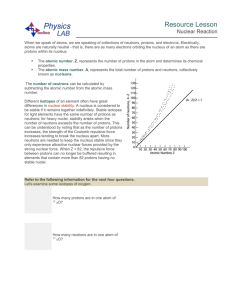
Resource Lesson Nuclear Reaction When we speak of atoms, we
... The number of neutrons can be calculated by subtracting the atomic number from the atomic mass number. Different isotopes of an element often have great differences in nuclear stability. A nucleus is considered to be stable if it remains together indefinitely. Stable isotopes for light elements have ...
... The number of neutrons can be calculated by subtracting the atomic number from the atomic mass number. Different isotopes of an element often have great differences in nuclear stability. A nucleus is considered to be stable if it remains together indefinitely. Stable isotopes for light elements have ...
Chemistry: Nuclear Reactions Guided Inquiry + n → + + 3 n +
... Nuclear reactions are reactions that affect the nucleus of an atom. In nature, unstable nuclei undergo nuclear reactions to form more stable nuclei. Stable nuclei can also undergo nuclear reactions if ...
... Nuclear reactions are reactions that affect the nucleus of an atom. In nature, unstable nuclei undergo nuclear reactions to form more stable nuclei. Stable nuclei can also undergo nuclear reactions if ...
PowerPoint 演示文稿
... While uranium-235 is the naturally occuring fissionable isotope, there are other isotopes which can be induced to fission by neutron bombardment. Plutonium-239 is also fissionable by bombardment with slow neutrons, and both it and uranium-235 have been used to make nuclear fission bombs. Plutonium-2 ...
... While uranium-235 is the naturally occuring fissionable isotope, there are other isotopes which can be induced to fission by neutron bombardment. Plutonium-239 is also fissionable by bombardment with slow neutrons, and both it and uranium-235 have been used to make nuclear fission bombs. Plutonium-2 ...
Nuclear Radiation and Decay File
... atomic mass that is the same as the original element. • Because the atom now has one more proton, it becomes the element with an atomic number one greater than that of the original element. ...
... atomic mass that is the same as the original element. • Because the atom now has one more proton, it becomes the element with an atomic number one greater than that of the original element. ...
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is either a nuclear reaction or a radioactive decay process in which the nucleus of an atom splits into smaller parts (lighter nuclei). The fission process often produces free neutrons and photons (in the form of gamma rays), and releases a very large amount of energy even by the energetic standards of radioactive decay.Nuclear fission of heavy elements was discovered on December 17, 1938 by German Otto Hahn and his assistant Fritz Strassmann, and explained theoretically in January 1939 by Lise Meitner and her nephew Otto Robert Frisch. Frisch named the process by analogy with biological fission of living cells. It is an exothermic reaction which can release large amounts of energy both as electromagnetic radiation and as kinetic energy of the fragments (heating the bulk material where fission takes place). In order for fission to produce energy, the total binding energy of the resulting elements must be less negative (higher energy) than that of the starting element.Fission is a form of nuclear transmutation because the resulting fragments are not the same element as the original atom. The two nuclei produced are most often of comparable but slightly different sizes, typically with a mass ratio of products of about 3 to 2, for common fissile isotopes. Most fissions are binary fissions (producing two charged fragments), but occasionally (2 to 4 times per 1000 events), three positively charged fragments are produced, in a ternary fission. The smallest of these fragments in ternary processes ranges in size from a proton to an argon nucleus.Apart from fission induced by a neutron, harnessed and exploited by humans, a natural form of spontaneous radioactive decay (not requiring a neutron) is also referred to as fission, and occurs especially in very high-mass-number isotopes. Spontaneous fission was discovered in 1940 by Flyorov, Petrzhak and Kurchatov in Moscow, when they decided to confirm that, without bombardment by neutrons, the fission rate of uranium was indeed negligible, as predicted by Niels Bohr; it wasn't.The unpredictable composition of the products (which vary in a broad probabilistic and somewhat chaotic manner) distinguishes fission from purely quantum-tunnelling processes such as proton emission, alpha decay and cluster decay, which give the same products each time. Nuclear fission produces energy for nuclear power and drives the explosion of nuclear weapons. Both uses are possible because certain substances called nuclear fuels undergo fission when struck by fission neutrons, and in turn emit neutrons when they break apart. This makes possible a self-sustaining nuclear chain reaction that releases energy at a controlled rate in a nuclear reactor or at a very rapid uncontrolled rate in a nuclear weapon.The amount of free energy contained in nuclear fuel is millions of times the amount of free energy contained in a similar mass of chemical fuel such as gasoline, making nuclear fission a very dense source of energy. The products of nuclear fission, however, are on average far more radioactive than the heavy elements which are normally fissioned as fuel, and remain so for significant amounts of time, giving rise to a nuclear waste problem. Concerns over nuclear waste accumulation and over the destructive potential of nuclear weapons may counterbalance the desirable qualities of fission as an energy source, and give rise to ongoing political debate over nuclear power.