Article 2: Key Concepts and Vocabulary
... most of the electricity is sent on transmission lines to provide consumers with power for refrigerators, lights, computers, and many other devices. Although amounts of energy are expressed in many different units, depending on the country and the context, the scientific community uses a unified syst ...
... most of the electricity is sent on transmission lines to provide consumers with power for refrigerators, lights, computers, and many other devices. Although amounts of energy are expressed in many different units, depending on the country and the context, the scientific community uses a unified syst ...
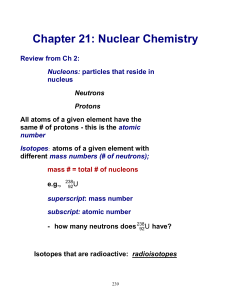
Chapter 21: Nuclear Chemistry
... When a nucleus spontaneously decomposes in this way, it is said to have undergone radioactive decay Note that radioactive properties are independent of the state of chemical combination of an atom – we are not concerned with whether the atom is in the form of an element or in a compound ...
... When a nucleus spontaneously decomposes in this way, it is said to have undergone radioactive decay Note that radioactive properties are independent of the state of chemical combination of an atom – we are not concerned with whether the atom is in the form of an element or in a compound ...
ENERGY IN THE NUCLEUS OF THE ATOM
... Energy (E) is equal to mass (m) times some constant (c is the speed of light) squared. What it means is that mass is energy, and vice-versa. ...
... Energy (E) is equal to mass (m) times some constant (c is the speed of light) squared. What it means is that mass is energy, and vice-versa. ...
U - Earth and Environmental Sciences
... Uranium-238 is abundant in the nuclear "fuel"; this leads to the production of plutonium. Nuclear fusion has the potential to yield enormous amounts of energy if it can be done in a controlled, sustained way. Consider the mass-to-energy numbers... 2 protons = 2 x 1.00728 u = 2.01456 u 2 neutrons = 2 ...
... Uranium-238 is abundant in the nuclear "fuel"; this leads to the production of plutonium. Nuclear fusion has the potential to yield enormous amounts of energy if it can be done in a controlled, sustained way. Consider the mass-to-energy numbers... 2 protons = 2 x 1.00728 u = 2.01456 u 2 neutrons = 2 ...
nuclear fission
... nuclear power stations have been built around the world. The neutrons produced in a chain reaction are moving too fast to cause further fission in U235 nuclei and they have to be slowed down. This is done by graphite or heavy water and these materials are called moderators. As the neutrons collide w ...
... nuclear power stations have been built around the world. The neutrons produced in a chain reaction are moving too fast to cause further fission in U235 nuclei and they have to be slowed down. This is done by graphite or heavy water and these materials are called moderators. As the neutrons collide w ...
Scientists` Consensus Ideas Atomic Structure and Nuclear Interactions
... 14. Some elements change into other elements as a result of nuclear reactions. Physical and chemical interactions do not convert one element into another element. 15. Nuclear radiation refers to the particles and energy released during nuclear reactions. Three sources of nuclear radiation are radioa ...
... 14. Some elements change into other elements as a result of nuclear reactions. Physical and chemical interactions do not convert one element into another element. 15. Nuclear radiation refers to the particles and energy released during nuclear reactions. Three sources of nuclear radiation are radioa ...
atoms - Groupfusion.net
... Isotopes = atoms of the same element with the same number of protons but different number of neutrons. = same atomic number ; different mass number Almost all elements have isotopes. Some have many and some have only a few. The isotopes of an element always occur in the same percentage. Isotopes of ...
... Isotopes = atoms of the same element with the same number of protons but different number of neutrons. = same atomic number ; different mass number Almost all elements have isotopes. Some have many and some have only a few. The isotopes of an element always occur in the same percentage. Isotopes of ...
Content Domain III: Chemistry—Atomic Theory and
... isotopes, law of conservation of matter, matter, neutron, nucleus, proton, alpha particles, beta particles, fission, fusion, gamma radiation, half-life, radioactive decay. The current model suggests that an atom consists of a positively charged, small, dense central region surrounded by a negatively ...
... isotopes, law of conservation of matter, matter, neutron, nucleus, proton, alpha particles, beta particles, fission, fusion, gamma radiation, half-life, radioactive decay. The current model suggests that an atom consists of a positively charged, small, dense central region surrounded by a negatively ...
1 + - crypt
... What isotopes are used for fission reactions? Copy Figure 3 on page 203 explain how a nuclear fission reactor produces steam to drive the turbines of a power station. Your account should include the purpose of the moderator, control rods and coolant. Copy and answer question (b) on page 203. Copy th ...
... What isotopes are used for fission reactions? Copy Figure 3 on page 203 explain how a nuclear fission reactor produces steam to drive the turbines of a power station. Your account should include the purpose of the moderator, control rods and coolant. Copy and answer question (b) on page 203. Copy th ...
Chapter 25 Radioactivity
... Some nuclei are stable, some are unstable Larger nucleus = more unstable Smaller nucleus = more stable The nucleus of an atom is like a marble in the center of a football arena. The atom is composed of mostly space ...
... Some nuclei are stable, some are unstable Larger nucleus = more unstable Smaller nucleus = more stable The nucleus of an atom is like a marble in the center of a football arena. The atom is composed of mostly space ...
AP Chem
... even number of neutrons. The least stable situation is when both numbers are odd. There are only four (or five) stable odd/odd nuclei. Nuclides with a mass number over 200 usually undergo alpha decay. They emit a particle consisting of two protons and two neutrons. Nuclides with too many neutrons un ...
... even number of neutrons. The least stable situation is when both numbers are odd. There are only four (or five) stable odd/odd nuclei. Nuclides with a mass number over 200 usually undergo alpha decay. They emit a particle consisting of two protons and two neutrons. Nuclides with too many neutrons un ...
Radioactivity
... Electron Capture • If there are too many protons in a nucleus, it may capture an electron • A proton becomes a neutron ...
... Electron Capture • If there are too many protons in a nucleus, it may capture an electron • A proton becomes a neutron ...
Radioactivity
... Nuclear Stability • Nuclear particles (protons and neutrons) are called nucleons • Nucleons are held together by nuclear strong force (short range, very strong) • Neutrons are “glue” – necessary to hold the nucleus together • Without neutrons the nucleus would fly apart due to electrostatic repuls ...
... Nuclear Stability • Nuclear particles (protons and neutrons) are called nucleons • Nucleons are held together by nuclear strong force (short range, very strong) • Neutrons are “glue” – necessary to hold the nucleus together • Without neutrons the nucleus would fly apart due to electrostatic repuls ...
Click here for printer-friendly sample test questions
... A. are responsible for the formation of most elements. B. are commonly used in nuclear power plants. C. are used in Russian-style nuclear reactors. D. occur when electrons combine with neutrons. 3. Nuclear fission reactions A. are responsible for the formation of most elements. B. are commonly used ...
... A. are responsible for the formation of most elements. B. are commonly used in nuclear power plants. C. are used in Russian-style nuclear reactors. D. occur when electrons combine with neutrons. 3. Nuclear fission reactions A. are responsible for the formation of most elements. B. are commonly used ...
L37 - University of Iowa Physics
... holds the atom together • the neutrons and protons have about the same mass, and are each about 2000 times more massive than the electrons • the nucleus accounts for about 99.9% of the total mass of the atom • the neutrons have no charge what role ...
... holds the atom together • the neutrons and protons have about the same mass, and are each about 2000 times more massive than the electrons • the nucleus accounts for about 99.9% of the total mass of the atom • the neutrons have no charge what role ...
Syllabus overview
... 3.1.1 State that temperature determines the direction of thermal energy transfer between two objects. Students should be familiar with the concept of thermal equilibrium. 3.1.2 State the relation between the Kelvin and Celsius scales of temperature. T/K = t/°C + 273 is sufficient. 3.1.3 State that t ...
... 3.1.1 State that temperature determines the direction of thermal energy transfer between two objects. Students should be familiar with the concept of thermal equilibrium. 3.1.2 State the relation between the Kelvin and Celsius scales of temperature. T/K = t/°C + 273 is sufficient. 3.1.3 State that t ...
Nuclear Radiation1516
... When a nucleus fissions, it splits into several smaller fragments. These fragments, or fission products, are about equal to half the original mass. Two or three neutrons are also emitted. The sum of the masses of these fragments is less than the original mass. This 'missing' mass (about 0.1 percent ...
... When a nucleus fissions, it splits into several smaller fragments. These fragments, or fission products, are about equal to half the original mass. Two or three neutrons are also emitted. The sum of the masses of these fragments is less than the original mass. This 'missing' mass (about 0.1 percent ...
Lecture 30/3 Nuclear processes Ulf Torkelsson 1 Nuclear reactions
... The nuclei that are involved in a fusion reaction are repelling each other through the Coulomb force, since they are both positively charged. This Coulomb repulsion is so strong that the kinetic energy of the nuclei is insufficient in bringing them any closer together than a thousand times the radiu ...
... The nuclei that are involved in a fusion reaction are repelling each other through the Coulomb force, since they are both positively charged. This Coulomb repulsion is so strong that the kinetic energy of the nuclei is insufficient in bringing them any closer together than a thousand times the radiu ...
Example 27-3 The Binding Energy of 4He
... binding energy of the nucleus divided by the number of nucleons in the nucleus. ...
... binding energy of the nucleus divided by the number of nucleons in the nucleus. ...
The Band of Stability
... distances. If there are too few neutrons, or too many neutrons, the nucleus becomes unstable. If an atom has more than 82 protons in the nucleus, there is no arrangement of neutrons that can produce more attractive forces than repulsive forces. Therefore, all isotopes of elements beyond lead are rad ...
... distances. If there are too few neutrons, or too many neutrons, the nucleus becomes unstable. If an atom has more than 82 protons in the nucleus, there is no arrangement of neutrons that can produce more attractive forces than repulsive forces. Therefore, all isotopes of elements beyond lead are rad ...
I Examen II trim Science
... • Stretches into an elongated shape • Electric forces push it into an even more elongated shape • Electric forces > strong nuclear forces • The nucleus splits • U-235 released energy (kinetic ENERGY, ejects a neutron and gamma radiation) • Creates a chain reaction within each nucleus separating. • N ...
... • Stretches into an elongated shape • Electric forces push it into an even more elongated shape • Electric forces > strong nuclear forces • The nucleus splits • U-235 released energy (kinetic ENERGY, ejects a neutron and gamma radiation) • Creates a chain reaction within each nucleus separating. • N ...
Fusion or Fission
... the heat required can exceed several million degrees Celsius (yes, several million!) To put these types of temperatures in perspective, the temperature at the surface of the sun is around 5,600 ºC. Meanwhile, the temperature near the core of the sun is calculated to be around 15 million ºC. The temp ...
... the heat required can exceed several million degrees Celsius (yes, several million!) To put these types of temperatures in perspective, the temperature at the surface of the sun is around 5,600 ºC. Meanwhile, the temperature near the core of the sun is calculated to be around 15 million ºC. The temp ...
Nuclear Physics and Radioactivity
... alpha particle - positively charged particle consisting of two protons and two neutrons. (Helium nucleus) atomic mass number (A) - the number of protons and neutrons in the nucleus of an atom. atomic mass unit - the unit of mass equal to 1/12 the mass of a carbon-12 nucleus; the atomic mass rounded ...
... alpha particle - positively charged particle consisting of two protons and two neutrons. (Helium nucleus) atomic mass number (A) - the number of protons and neutrons in the nucleus of an atom. atomic mass unit - the unit of mass equal to 1/12 the mass of a carbon-12 nucleus; the atomic mass rounded ...
Radioactivity - Teach Nuclear
... Atomic number increases by 1 (new element) During this conversion an electron and an antineutrino are ejected from the nucleus ...
... Atomic number increases by 1 (new element) During this conversion an electron and an antineutrino are ejected from the nucleus ...
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is either a nuclear reaction or a radioactive decay process in which the nucleus of an atom splits into smaller parts (lighter nuclei). The fission process often produces free neutrons and photons (in the form of gamma rays), and releases a very large amount of energy even by the energetic standards of radioactive decay.Nuclear fission of heavy elements was discovered on December 17, 1938 by German Otto Hahn and his assistant Fritz Strassmann, and explained theoretically in January 1939 by Lise Meitner and her nephew Otto Robert Frisch. Frisch named the process by analogy with biological fission of living cells. It is an exothermic reaction which can release large amounts of energy both as electromagnetic radiation and as kinetic energy of the fragments (heating the bulk material where fission takes place). In order for fission to produce energy, the total binding energy of the resulting elements must be less negative (higher energy) than that of the starting element.Fission is a form of nuclear transmutation because the resulting fragments are not the same element as the original atom. The two nuclei produced are most often of comparable but slightly different sizes, typically with a mass ratio of products of about 3 to 2, for common fissile isotopes. Most fissions are binary fissions (producing two charged fragments), but occasionally (2 to 4 times per 1000 events), three positively charged fragments are produced, in a ternary fission. The smallest of these fragments in ternary processes ranges in size from a proton to an argon nucleus.Apart from fission induced by a neutron, harnessed and exploited by humans, a natural form of spontaneous radioactive decay (not requiring a neutron) is also referred to as fission, and occurs especially in very high-mass-number isotopes. Spontaneous fission was discovered in 1940 by Flyorov, Petrzhak and Kurchatov in Moscow, when they decided to confirm that, without bombardment by neutrons, the fission rate of uranium was indeed negligible, as predicted by Niels Bohr; it wasn't.The unpredictable composition of the products (which vary in a broad probabilistic and somewhat chaotic manner) distinguishes fission from purely quantum-tunnelling processes such as proton emission, alpha decay and cluster decay, which give the same products each time. Nuclear fission produces energy for nuclear power and drives the explosion of nuclear weapons. Both uses are possible because certain substances called nuclear fuels undergo fission when struck by fission neutrons, and in turn emit neutrons when they break apart. This makes possible a self-sustaining nuclear chain reaction that releases energy at a controlled rate in a nuclear reactor or at a very rapid uncontrolled rate in a nuclear weapon.The amount of free energy contained in nuclear fuel is millions of times the amount of free energy contained in a similar mass of chemical fuel such as gasoline, making nuclear fission a very dense source of energy. The products of nuclear fission, however, are on average far more radioactive than the heavy elements which are normally fissioned as fuel, and remain so for significant amounts of time, giving rise to a nuclear waste problem. Concerns over nuclear waste accumulation and over the destructive potential of nuclear weapons may counterbalance the desirable qualities of fission as an energy source, and give rise to ongoing political debate over nuclear power.