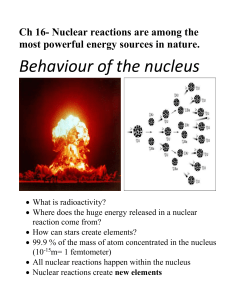
Atomic Energy for Military Purposes
... before discussing the laws that govern such emission, we shall describe the current ideas on how atoms are constructed, ideas based partly on the study of radioactivity. 1.11. According to our present view every atom consists of a smallheavy nucleus approximately 10-12 cm in diameter surrounded by a ...
... before discussing the laws that govern such emission, we shall describe the current ideas on how atoms are constructed, ideas based partly on the study of radioactivity. 1.11. According to our present view every atom consists of a smallheavy nucleus approximately 10-12 cm in diameter surrounded by a ...
Period 10 Activity Solutions: Nuclear Reactions
... half as radioactive and the number of the original nuclei remaining will be only half what it was originally. After 2 half-lives, only one fourth of the original radioactive material will remain. 2) Carbon-14 a) What common radioactive isotope is used for archeological dating? The carbon-14 isotope ...
... half as radioactive and the number of the original nuclei remaining will be only half what it was originally. After 2 half-lives, only one fourth of the original radioactive material will remain. 2) Carbon-14 a) What common radioactive isotope is used for archeological dating? The carbon-14 isotope ...
entc 4390 medical imaging
... Actual particles that are produced during the decay of certain radioacitve materials. ...
... Actual particles that are produced during the decay of certain radioacitve materials. ...
Lecture - 1
... highly energetic compared to chemical reactions. For example, fission of an atom of Uranium-235 releases about 210 MeV of energy, while energy released due to the formation of one molecule of CO2 from the combustion of carbon is about 4.08 eV. The types of nuclear reactions that are of importance in ...
... highly energetic compared to chemical reactions. For example, fission of an atom of Uranium-235 releases about 210 MeV of energy, while energy released due to the formation of one molecule of CO2 from the combustion of carbon is about 4.08 eV. The types of nuclear reactions that are of importance in ...
Chapter 37
... • Radioactivity was discovered by Antoine Henri Becquerel in 1896. – The work involved uranium salts which lead to the conclusion that the minerals gave off some sort of radiation. – This radiation was later shown to be separable by electric (and magnetic) fields into three types; alpha (a), beta (b ...
... • Radioactivity was discovered by Antoine Henri Becquerel in 1896. – The work involved uranium salts which lead to the conclusion that the minerals gave off some sort of radiation. – This radiation was later shown to be separable by electric (and magnetic) fields into three types; alpha (a), beta (b ...
Radioactivity Revision Questions Decay – Nucleus
... ray. After an alpha particle or a beta particle has been emitted from a nucleus, the atomic number of the atom will have changed. The atom will have changed into a different element. An alpha or beta emitter might also emit gamma radiation. 5. What does the Nucleus of an Atom contain? The nucleus of ...
... ray. After an alpha particle or a beta particle has been emitted from a nucleus, the atomic number of the atom will have changed. The atom will have changed into a different element. An alpha or beta emitter might also emit gamma radiation. 5. What does the Nucleus of an Atom contain? The nucleus of ...
Unit 3 - Princeton High School
... the atoms in a sample to undergo decay. Assume that a 64-gram sample of Cs-137 is analyzed every 30 years for a 150 –year period. Calculate the grams of cesium and barium present each time the sample is analyzed and record the data in the table below. Note how the fraction remaining of the original ...
... the atoms in a sample to undergo decay. Assume that a 64-gram sample of Cs-137 is analyzed every 30 years for a 150 –year period. Calculate the grams of cesium and barium present each time the sample is analyzed and record the data in the table below. Note how the fraction remaining of the original ...
By what process do most stars release energy? A. Electromagnetic
... Nuclear energy can be generated by ssion or fusion. Fusion is not currently being used in reactors as an energy source. Why is this? A. ...
... Nuclear energy can be generated by ssion or fusion. Fusion is not currently being used in reactors as an energy source. Why is this? A. ...
Radioactive decay of nucleus
... 7. In an experiment, a researcher studied the decay of Po-210, which decays by the alpha emission and releases a stable Pb-206 atom. The half-life of Po-210 is 138.4 days. The mass of the Po-210 sample at the start of the experiment was 34.0g. (a)Write an equation for the alpha-decay of Po-210 (b)Wh ...
... 7. In an experiment, a researcher studied the decay of Po-210, which decays by the alpha emission and releases a stable Pb-206 atom. The half-life of Po-210 is 138.4 days. The mass of the Po-210 sample at the start of the experiment was 34.0g. (a)Write an equation for the alpha-decay of Po-210 (b)Wh ...
Nuclear_Chem_016
... nuclides would be left after 360 days? 2) A medical institution requests 1 g of bismuth-214, which has a half life of 20 min. How many grams of bismuth-214 must be prepared if the shipping time is 2 h? 3) Use reference table to write the nuclear equation for the decay of iodine 131. What particle is ...
... nuclides would be left after 360 days? 2) A medical institution requests 1 g of bismuth-214, which has a half life of 20 min. How many grams of bismuth-214 must be prepared if the shipping time is 2 h? 3) Use reference table to write the nuclear equation for the decay of iodine 131. What particle is ...
Mindfiesta Page 1 CHAPTER – 13 NUCLEI EXPERT`S TIPS : (1) An
... (53) After a time t (= n T), the fraction of the radioactive atoms left behind, n ...
... (53) After a time t (= n T), the fraction of the radioactive atoms left behind, n ...
Alpha Decay
... The alpha particle is a helium nucleus (2protons, 2 neutrons) produced from the radioactive decay of heavy metals and some nuclear reaction. The high positive charge (2+) of an alpha particle causes electrical excitation and ionization of surrounding atoms. Alpha particles are the least penetr ...
... The alpha particle is a helium nucleus (2protons, 2 neutrons) produced from the radioactive decay of heavy metals and some nuclear reaction. The high positive charge (2+) of an alpha particle causes electrical excitation and ionization of surrounding atoms. Alpha particles are the least penetr ...
Chapter 16 Notes - Mr. Julien`s Homepage
... produces an exposed photographic plate. 3. Areas of decreased or increased radiation can indicate a disease, a tumor, a blood clot or an edema. 4. Ingestion of I-131 can be used to see how much iodine is taken in by a thyroid gland which can indicate hyperthyroidism if more is taken in than normal. ...
... produces an exposed photographic plate. 3. Areas of decreased or increased radiation can indicate a disease, a tumor, a blood clot or an edema. 4. Ingestion of I-131 can be used to see how much iodine is taken in by a thyroid gland which can indicate hyperthyroidism if more is taken in than normal. ...
Chapter 11 Notes
... So each organism is continuously replenishing the amount of C-14 in its body. Once the organism dies, no more C-14 is consumed so the level of C-14 begins to ...
... So each organism is continuously replenishing the amount of C-14 in its body. Once the organism dies, no more C-14 is consumed so the level of C-14 begins to ...
Nuclear Chemistry
... stable nuclei are odd-odd. (This can't be seen from this graph due to its small size and lack of detail.) ...
... stable nuclei are odd-odd. (This can't be seen from this graph due to its small size and lack of detail.) ...
Nuclear Processes
... When a radioactive nucleus such as U23892 decays it often produces another radioactive isotope which goes on to decay further. ...
... When a radioactive nucleus such as U23892 decays it often produces another radioactive isotope which goes on to decay further. ...
radioactive decay
... Neutron-to-proton (n/p) ratio and nuclear stability. To some degree, the stability of a nucleus can be correlated with its neutron-to-proton ratio. For light atoms 1/1 ratio, for heavier atoms closer ot 1.5/1 ...
... Neutron-to-proton (n/p) ratio and nuclear stability. To some degree, the stability of a nucleus can be correlated with its neutron-to-proton ratio. For light atoms 1/1 ratio, for heavier atoms closer ot 1.5/1 ...
File
... Some isotopes are stable and happy. These are the elements that we see around us and find in nature. Stable atoms generally have the same number of protons and neutrons. Some accommodate one to two additional neutrons in the nucleus and remain stable. However, when the nucleus of an atom possesses e ...
... Some isotopes are stable and happy. These are the elements that we see around us and find in nature. Stable atoms generally have the same number of protons and neutrons. Some accommodate one to two additional neutrons in the nucleus and remain stable. However, when the nucleus of an atom possesses e ...
7.3 – Nuclear Reactions, Fission and Fusion 7.3.1 – Describe and
... 7.3.3 – Define the term unified atomic mass unit Students must be familiar with the units MeV c -2 and GeV c -2 for mass 7.3.4 - Apply the Einstein mass – energy equivalence relationship 7.3.5 – Define the concepts of mass defect, binding energy and binding energy per nucleon 7.3.6 – Draw and annota ...
... 7.3.3 – Define the term unified atomic mass unit Students must be familiar with the units MeV c -2 and GeV c -2 for mass 7.3.4 - Apply the Einstein mass – energy equivalence relationship 7.3.5 – Define the concepts of mass defect, binding energy and binding energy per nucleon 7.3.6 – Draw and annota ...
nuclear physics - Sakshi Education
... The radioactivity of a sample is ‘X’ at a time ‘t 1 ’ and ‘Y’ at a time ‘t 2 ’. If the mean life time of the specimen is τ , the number of atoms that have disintegrated in the time interval (t 1 - t 2 ) is : (2009 E) ...
... The radioactivity of a sample is ‘X’ at a time ‘t 1 ’ and ‘Y’ at a time ‘t 2 ’. If the mean life time of the specimen is τ , the number of atoms that have disintegrated in the time interval (t 1 - t 2 ) is : (2009 E) ...
Waves notes section 5 - Nuclear radiation
... 1. The fuel rods are made of uranium which produces energy by fission. 2. The moderator, normally made of graphite slows down neutrons that are produced in fission, since a nucleus is split more easily by slow moving neutrons. 3. The control rods are made of boron, and absorb neutrons when lowered ...
... 1. The fuel rods are made of uranium which produces energy by fission. 2. The moderator, normally made of graphite slows down neutrons that are produced in fission, since a nucleus is split more easily by slow moving neutrons. 3. The control rods are made of boron, and absorb neutrons when lowered ...
Chapter 3 Nuclear Radiation
... A radioactive isotope • has an unstable nucleus. • emits radiation to become more stable. • can be one or more of the isotopes of an element ...
... A radioactive isotope • has an unstable nucleus. • emits radiation to become more stable. • can be one or more of the isotopes of an element ...
Adobe Acrobat file () - Wayne State University Physics and
... number of nucleons as the parent, but the atomic number is one less In addition, an electron (positron) was observed The emission of the electron is from the nucleus ...
... number of nucleons as the parent, but the atomic number is one less In addition, an electron (positron) was observed The emission of the electron is from the nucleus ...
Chapter 3 Nuclear Radiation
... A radioactive isotope • has an unstable nucleus. • emits radiation to become more stable. • can be one or more of the isotopes of an element ...
... A radioactive isotope • has an unstable nucleus. • emits radiation to become more stable. • can be one or more of the isotopes of an element ...
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is either a nuclear reaction or a radioactive decay process in which the nucleus of an atom splits into smaller parts (lighter nuclei). The fission process often produces free neutrons and photons (in the form of gamma rays), and releases a very large amount of energy even by the energetic standards of radioactive decay.Nuclear fission of heavy elements was discovered on December 17, 1938 by German Otto Hahn and his assistant Fritz Strassmann, and explained theoretically in January 1939 by Lise Meitner and her nephew Otto Robert Frisch. Frisch named the process by analogy with biological fission of living cells. It is an exothermic reaction which can release large amounts of energy both as electromagnetic radiation and as kinetic energy of the fragments (heating the bulk material where fission takes place). In order for fission to produce energy, the total binding energy of the resulting elements must be less negative (higher energy) than that of the starting element.Fission is a form of nuclear transmutation because the resulting fragments are not the same element as the original atom. The two nuclei produced are most often of comparable but slightly different sizes, typically with a mass ratio of products of about 3 to 2, for common fissile isotopes. Most fissions are binary fissions (producing two charged fragments), but occasionally (2 to 4 times per 1000 events), three positively charged fragments are produced, in a ternary fission. The smallest of these fragments in ternary processes ranges in size from a proton to an argon nucleus.Apart from fission induced by a neutron, harnessed and exploited by humans, a natural form of spontaneous radioactive decay (not requiring a neutron) is also referred to as fission, and occurs especially in very high-mass-number isotopes. Spontaneous fission was discovered in 1940 by Flyorov, Petrzhak and Kurchatov in Moscow, when they decided to confirm that, without bombardment by neutrons, the fission rate of uranium was indeed negligible, as predicted by Niels Bohr; it wasn't.The unpredictable composition of the products (which vary in a broad probabilistic and somewhat chaotic manner) distinguishes fission from purely quantum-tunnelling processes such as proton emission, alpha decay and cluster decay, which give the same products each time. Nuclear fission produces energy for nuclear power and drives the explosion of nuclear weapons. Both uses are possible because certain substances called nuclear fuels undergo fission when struck by fission neutrons, and in turn emit neutrons when they break apart. This makes possible a self-sustaining nuclear chain reaction that releases energy at a controlled rate in a nuclear reactor or at a very rapid uncontrolled rate in a nuclear weapon.The amount of free energy contained in nuclear fuel is millions of times the amount of free energy contained in a similar mass of chemical fuel such as gasoline, making nuclear fission a very dense source of energy. The products of nuclear fission, however, are on average far more radioactive than the heavy elements which are normally fissioned as fuel, and remain so for significant amounts of time, giving rise to a nuclear waste problem. Concerns over nuclear waste accumulation and over the destructive potential of nuclear weapons may counterbalance the desirable qualities of fission as an energy source, and give rise to ongoing political debate over nuclear power.