... questions regarding the class materials or you are asked any questions by the Instructor. Private talking in the class will hurt your understanding and also will hurt others. No sleeping in the class room allowed Objectives of the course: The principal objectives of this course is to get familiar wi ...
Objective Reaction Type Structural Feature How to figure out how reactants react?
... sulfhydryl-containing enzymes such as those found in the eye are a prime target, the Army says. Effects of CS exposure include an extreme burning sensation in the eyes with a copious flow of tears, coughing, sneezing, a perception of chest tightness, and dizziness. Most of these effects subside with ...
... sulfhydryl-containing enzymes such as those found in the eye are a prime target, the Army says. Effects of CS exposure include an extreme burning sensation in the eyes with a copious flow of tears, coughing, sneezing, a perception of chest tightness, and dizziness. Most of these effects subside with ...
1-13 acids esters fats
... These molecules are more polar than alcohols as they have a more polar region and they can also form hydrogen bonds because of the oxygen bonded to the hydrogen. The Melting point and boiling points and solubility are therefore much higher. Like alcohols the solubility diminishes as the molecule get ...
... These molecules are more polar than alcohols as they have a more polar region and they can also form hydrogen bonds because of the oxygen bonded to the hydrogen. The Melting point and boiling points and solubility are therefore much higher. Like alcohols the solubility diminishes as the molecule get ...
Organic Chemistry
... Most atoms are only capable of forming small molecules. However one or two can form larger molecules. By far and away the best atom for making large molecules with is Carbon. Carbon can make molecules that have tens, hundreds, thousands even millions of atoms! The huge number of possible combination ...
... Most atoms are only capable of forming small molecules. However one or two can form larger molecules. By far and away the best atom for making large molecules with is Carbon. Carbon can make molecules that have tens, hundreds, thousands even millions of atoms! The huge number of possible combination ...
How to Name Chemical Compounds
... Inorganic acids – these compounds contain hydrogen available for donation in a chemical reaction; their formulas generally start with H (hydrogen) and are followed by a nonmetal or polyatomic anion Hydrated compounds – these compounds appear as a salt or ionic compound with water molecules attached; ...
... Inorganic acids – these compounds contain hydrogen available for donation in a chemical reaction; their formulas generally start with H (hydrogen) and are followed by a nonmetal or polyatomic anion Hydrated compounds – these compounds appear as a salt or ionic compound with water molecules attached; ...
A Guide to Organic Molecules
... hydrocarbons highlighting their differences and their nomenclature. Lesson 3 goes further to introduce branched alkanes and isomerism. The IUPAC rules are highlighted in the naming of the branched alkanes. Lesson 3 also introduces the homologous series of haloalkanes (alkyl halides). Lesson 4 introd ...
... hydrocarbons highlighting their differences and their nomenclature. Lesson 3 goes further to introduce branched alkanes and isomerism. The IUPAC rules are highlighted in the naming of the branched alkanes. Lesson 3 also introduces the homologous series of haloalkanes (alkyl halides). Lesson 4 introd ...
Functional Groups Notes
... Organic Chemistry Notes - Functional Groups Definition: What is a functional group? A functional group is a sequence of atoms within a parent molecule that exhibits characteristic and predictable chemical behavior. A particular functional group generally exhibits a particular type of behavior, regar ...
... Organic Chemistry Notes - Functional Groups Definition: What is a functional group? A functional group is a sequence of atoms within a parent molecule that exhibits characteristic and predictable chemical behavior. A particular functional group generally exhibits a particular type of behavior, regar ...
Slide 1
... Esters have strong, fragrant aromas and are the cause of the odors and flavors of pineapple, banana, wintergreen and oranges. Ex. The smell of pineapples is due to ethyl butyrate ethanol + butanoic acid water (alcohol) ...
... Esters have strong, fragrant aromas and are the cause of the odors and flavors of pineapple, banana, wintergreen and oranges. Ex. The smell of pineapples is due to ethyl butyrate ethanol + butanoic acid water (alcohol) ...
Biology II Honors Chapter 4 Carbon and Molecular Diversity Guided
... • Is polar as a result of the electrons spending more time near the electronegative oxygen atom. • Can form hydrogen bonds with water molecules, helping dissolve organic compounds such as sugars. ...
... • Is polar as a result of the electrons spending more time near the electronegative oxygen atom. • Can form hydrogen bonds with water molecules, helping dissolve organic compounds such as sugars. ...
ORGANIC CONVERSION---(2 to 3 marks)
... (c) Reaction of propanone with methylmagnesium bromide followed by hydrolysis. # Give equations of the following reactions: (i) Oxidation of propan-1-ol with alkaline KMnO4 solution. (ii) Bromine in CS2 with phenol. (iii) Dilute HNO3 with phenol. # Explain the following with an example. Write equati ...
... (c) Reaction of propanone with methylmagnesium bromide followed by hydrolysis. # Give equations of the following reactions: (i) Oxidation of propan-1-ol with alkaline KMnO4 solution. (ii) Bromine in CS2 with phenol. (iii) Dilute HNO3 with phenol. # Explain the following with an example. Write equati ...
CCCH110D Inorganic vs Organic NOTES
... right. Some examples of biological molecules that incorporate the benzene ring: ...
... right. Some examples of biological molecules that incorporate the benzene ring: ...
Summary of Organic Compounds -Functional Groups and Reactions
... Hydrogen (hydrogenation) to form alcohols Controlled oxidation of aldehydes to form carboxylic acids Carboxylic acid + alcohol = ester + H2O ...
... Hydrogen (hydrogenation) to form alcohols Controlled oxidation of aldehydes to form carboxylic acids Carboxylic acid + alcohol = ester + H2O ...
Alcohols Phenols and Ethers
... bond and one for the hydroxyl group. The –ol suffix is last and takes precedence in the numbering. 3. If the hydroxyl group is directly attached to an aromatic ring, the compound is named as a phenol. 4. If the hydroxyl group occurs in a carboxylic acid, aldehyde, or ketone, it is named as a substit ...
... bond and one for the hydroxyl group. The –ol suffix is last and takes precedence in the numbering. 3. If the hydroxyl group is directly attached to an aromatic ring, the compound is named as a phenol. 4. If the hydroxyl group occurs in a carboxylic acid, aldehyde, or ketone, it is named as a substit ...
Bluing Components and Other Pigments of Boletes
... auriflammeus, which contains five more oxidized or chlorinated derivatives of vulpinic acid (Scheme 2). (Elkmont, Great Smoky Mountain National Park, NC) Scheme 2. Outline of biosynthetic pathways to produce the bolete pigments atromentin, grevillin A, and pulvinic acid derivatives (Gill and Steglic ...
... auriflammeus, which contains five more oxidized or chlorinated derivatives of vulpinic acid (Scheme 2). (Elkmont, Great Smoky Mountain National Park, NC) Scheme 2. Outline of biosynthetic pathways to produce the bolete pigments atromentin, grevillin A, and pulvinic acid derivatives (Gill and Steglic ...
Name Class Date 23.4 Polymers Organic compounds can bond
... Addition Polymers An addition polymer forms when unsaturated monomers covalently bond to form a long chain. The physical properties of polymers change with the length of the carbon chain. Polymers of ethylene, propylene, styrene, and others have many industrial uses. Addition polymers are widely use ...
... Addition Polymers An addition polymer forms when unsaturated monomers covalently bond to form a long chain. The physical properties of polymers change with the length of the carbon chain. Polymers of ethylene, propylene, styrene, and others have many industrial uses. Addition polymers are widely use ...
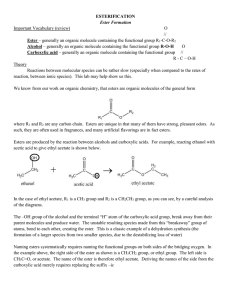
ESTERIFICATION Ester Formation Important Vocabulary (review) O
... In the case of ethyl acetate, R1 is a CH3 group and R2 is a CH2CH3 group, as you can see, by a careful analysis of the diagrams. The –OH group of the alcohol and the terminal “H” atom of the carboxylic acid group, break away from their parent molecules and produce water. The unstable resulting speci ...
... In the case of ethyl acetate, R1 is a CH3 group and R2 is a CH2CH3 group, as you can see, by a careful analysis of the diagrams. The –OH group of the alcohol and the terminal “H” atom of the carboxylic acid group, break away from their parent molecules and produce water. The unstable resulting speci ...
12.1 Alcohols: Structure and Physical Properties
... • Primary alcohols usually oxidize to carboxylic acids • With some care (using CrO3 as the reagent) an aldehyde may be obtained ...
... • Primary alcohols usually oxidize to carboxylic acids • With some care (using CrO3 as the reagent) an aldehyde may be obtained ...
Organic Chemistry PPT including assignments File
... the functional group that makes it a part of each group, state whether or not each is either saturated, unsaturated, or a substituted hydrocarbon, give an example of each to include name and ...
... the functional group that makes it a part of each group, state whether or not each is either saturated, unsaturated, or a substituted hydrocarbon, give an example of each to include name and ...
Unit 1 Test: Organic Chemistry Name
... d. Potassium permanganate (KMnO4) can also be used to test for unsaturated hydrocarbons. If KMnO4(aq) is added to ethane, the colour of the KMnO4 solution changes from purple to clear. What type of reaction has occurred? (1 mark) ______________________________________________________________________ ...
... d. Potassium permanganate (KMnO4) can also be used to test for unsaturated hydrocarbons. If KMnO4(aq) is added to ethane, the colour of the KMnO4 solution changes from purple to clear. What type of reaction has occurred? (1 mark) ______________________________________________________________________ ...
Chapter 17 – An Introduction to Organic Chemistry
... 24. All the polysaccharides are polymers, a general name for large molecules composed of repeating units, called monomers. 26. Protein molecules are polymers composed of monomers called amino acids. 28. A chemical reaction in which two substances combine to form a larger molecule with the release of ...
... 24. All the polysaccharides are polymers, a general name for large molecules composed of repeating units, called monomers. 26. Protein molecules are polymers composed of monomers called amino acids. 28. A chemical reaction in which two substances combine to form a larger molecule with the release of ...
Study Guide Chapter 17: An Introduction to Organic Chemistry
... To describe the different ways that organic molecules can be represented and show you how to convert from one way to the others. To show how you can recognize different types of organic compounds. There are millions of different organic (carbon-based) compounds. The task of studying them becomes ...
... To describe the different ways that organic molecules can be represented and show you how to convert from one way to the others. To show how you can recognize different types of organic compounds. There are millions of different organic (carbon-based) compounds. The task of studying them becomes ...
(a) Structural isomers
... • Isomers are compounds with the same molecular formula but different structures and properties – Structural isomers have different covalent arrangements of their atoms – Cis-trans isomers have the same covalent bonds but differ in spatial ...
... • Isomers are compounds with the same molecular formula but different structures and properties – Structural isomers have different covalent arrangements of their atoms – Cis-trans isomers have the same covalent bonds but differ in spatial ...
Chemistry of the earthy odour of basidiomata of Cortinarius hinnuleus
... Compounds 1–8 represent 96 % of total volatiles in C. hinnuleus. In particular, the volatiles 2–6 are responsible for the typical mushroom smell of fungi and have been known for some time (e.g. BELTRAN-GARCÍA & al. 1997, CHO & al. 2008, COMB & al. 2006, MALHEIRO & al. 2013). The occurrence of the al ...
... Compounds 1–8 represent 96 % of total volatiles in C. hinnuleus. In particular, the volatiles 2–6 are responsible for the typical mushroom smell of fungi and have been known for some time (e.g. BELTRAN-GARCÍA & al. 1997, CHO & al. 2008, COMB & al. 2006, MALHEIRO & al. 2013). The occurrence of the al ...
alcohol - Haverford Alchemy
... compounds by an oxidizing agent. • A carbonyl group is a carbon attached to an oxygen by a double bond. • Any oxidizing agent can be used. • In organic chemistry, a more general definition of oxidation and reduction is used. – An organic oxidation is one that increases the number of C—O bonds and/or ...
... compounds by an oxidizing agent. • A carbonyl group is a carbon attached to an oxygen by a double bond. • Any oxidizing agent can be used. • In organic chemistry, a more general definition of oxidation and reduction is used. – An organic oxidation is one that increases the number of C—O bonds and/or ...
Phenols

In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of a hydroxyl group (—OH) bonded directly to an aromatic hydrocarbon group. The simplest of the class is phenol, which is also called carbolic acid C6H5OH. Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule.Synonyms are arenols or aryl alcohols.Phenolic compounds are synthesized industrially; they also are produced by plants and microorganisms, with variation between and within species.Although similar to alcohols, phenols have unique properties and are not classified as alcohols (since the hydroxyl group is not bonded to a saturated carbon atom). They have higher acidities due to the aromatic ring's tight coupling with the oxygen and a relatively loose bond between the oxygen and hydrogen. The acidity of the hydroxyl group in phenols is commonly intermediate between that of aliphatic alcohols and carboxylic acids (their pKa is usually between 10 and 12).Loss of a positive hydrogen ion (H+) from the hydroxyl group of a phenol forms a corresponding negative phenolate ion or phenoxide ion, and the corresponding salts are called phenolates or phenoxides, although the term aryloxides is preferred according to the IUPAC Gold Book. Phenols can have two or more hydroxy groups bonded to the aromatic ring(s) in the same molecule. The simplest examples are the three benzenediols, each having two hydroxy groups on a benzene ring.Organisms that synthesize phenolic compounds do so in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research.ref name=Klepacka Some phenols are germicidal and are used in formulating disinfectants. Others possess estrogenic or endocrine disrupting activity.