PyMOL Modelling Workshop Outline of PyMOL usage
... There you will find links to download the latest educational version of PyMOL as well as a link to my “Introduction to PyMOL”, which in turn has links to other people's PyMOL tutorials. Note also the PyMOL Wiki: http://pymolwiki.org. Structure files can be found by searching the Protein Data Bank (P ...
... There you will find links to download the latest educational version of PyMOL as well as a link to my “Introduction to PyMOL”, which in turn has links to other people's PyMOL tutorials. Note also the PyMOL Wiki: http://pymolwiki.org. Structure files can be found by searching the Protein Data Bank (P ...
Thermodynamic and transport studies on some basic amino acids in
... number, apparent molal compressibility, apparent molal volume, limiting apparent molal compressibility, limiting apparent molal volume and the constants SK and SV and viscosity B-coefficient are given in Tables 2-4. Further, Figs 1-5 show the variations of adiabatic compressibility, molal hydration ...
... number, apparent molal compressibility, apparent molal volume, limiting apparent molal compressibility, limiting apparent molal volume and the constants SK and SV and viscosity B-coefficient are given in Tables 2-4. Further, Figs 1-5 show the variations of adiabatic compressibility, molal hydration ...
Electron attachment to molecular clusters by collisional charge transfer
... to the molecular cluster within the Coulombic field of the alkali cation. This obviates momentum transfer constraints. It also enables the attachment to occur isoenergetically at threshold. Fragmentation of the clusters is thereby minimized, which permits the electron affmity for an X, cluster to be ...
... to the molecular cluster within the Coulombic field of the alkali cation. This obviates momentum transfer constraints. It also enables the attachment to occur isoenergetically at threshold. Fragmentation of the clusters is thereby minimized, which permits the electron affmity for an X, cluster to be ...
FM 10-67-2 Chapter 7
... responsible to identify chemical substances (perform qualitative analysis) and estimate quantities present (perform quantitative analysis) in their examination of fuel samples. This chapter addresses some of the basic terms, formulas, tests, and equipmentthey will use. DEFINITION Matter is anything ...
... responsible to identify chemical substances (perform qualitative analysis) and estimate quantities present (perform quantitative analysis) in their examination of fuel samples. This chapter addresses some of the basic terms, formulas, tests, and equipmentthey will use. DEFINITION Matter is anything ...
CHEMISTRY
... 4. Students typically have problems distinguishing between chemical and physical changes. Frying an egg is an example that confuses students—they think it’s a physical change because of the change from liquid to solid. When I ask them how they usually make a liquid turn to a solid, their answer is t ...
... 4. Students typically have problems distinguishing between chemical and physical changes. Frying an egg is an example that confuses students—they think it’s a physical change because of the change from liquid to solid. When I ask them how they usually make a liquid turn to a solid, their answer is t ...
Title 1 Graft copolymerization of cellulose Student Synopsis
... enough supply of fish with the increase of population. However, one of the major constrain facing for aquaculture industry is increasing cost of production where one of them is price of fish feed [1]. Fish feed contains crude protein, crude lipid, ash, salt, ammonia-N, moisture, vitamin and antioxid ...
... enough supply of fish with the increase of population. However, one of the major constrain facing for aquaculture industry is increasing cost of production where one of them is price of fish feed [1]. Fish feed contains crude protein, crude lipid, ash, salt, ammonia-N, moisture, vitamin and antioxid ...
CHAPTER 4: CHEMICAL QUANTITIES and AQUEOUS REACTIONS
... the mass of water? 5. Write the equation CH3OH + O2 → CO2 + H2O 6. Balance the chemical equation 2CH3OH + 3O2 → 2CO2 + 4H2O 7. From the balanced equation, find out the moles of desired reactants and products. 2 moles of CH3OH gives 4 moles of H2O. 8. Convert moles to grams. Molar mass of CH3OH = (1 ...
... the mass of water? 5. Write the equation CH3OH + O2 → CO2 + H2O 6. Balance the chemical equation 2CH3OH + 3O2 → 2CO2 + 4H2O 7. From the balanced equation, find out the moles of desired reactants and products. 2 moles of CH3OH gives 4 moles of H2O. 8. Convert moles to grams. Molar mass of CH3OH = (1 ...
Stoichiometry: Calculations with Chemical
... Empirical to molecular • Since the empirical formula is the lowest ratio the actual molecule would weigh more. • By a whole number multiple. • Divide the actual molar mass by the the mass of one mole of the empirical formula. • Caffeine has a molar mass of 194 g. what is its molecular mass? ...
... Empirical to molecular • Since the empirical formula is the lowest ratio the actual molecule would weigh more. • By a whole number multiple. • Divide the actual molar mass by the the mass of one mole of the empirical formula. • Caffeine has a molar mass of 194 g. what is its molecular mass? ...
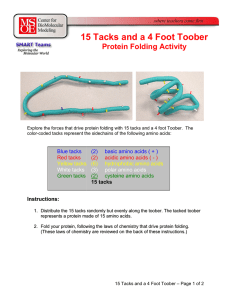
15 Tacks and a 4 Foot Toober
... globular protein, where they are hidden from polar water molecules. 2. Charged sidechains (blue and red tacks) will be on the surface of proteins where they often neutralize each other and form salt bridges. 3. Polar sidechains (white tacks) will be on the surface of the protein where they can hydro ...
... globular protein, where they are hidden from polar water molecules. 2. Charged sidechains (blue and red tacks) will be on the surface of proteins where they often neutralize each other and form salt bridges. 3. Polar sidechains (white tacks) will be on the surface of the protein where they can hydro ...
Student Solutions Manual Errata
... (a) To determine the limiting reactant, we can calculate the mass of F2 required to react with the specified mass of N2. If the calculated mass of F2 is greater than the available mass, then F2 is the limiting reactant. If the calculated mass is smaller than the available mass, then N2 is the limiti ...
... (a) To determine the limiting reactant, we can calculate the mass of F2 required to react with the specified mass of N2. If the calculated mass of F2 is greater than the available mass, then F2 is the limiting reactant. If the calculated mass is smaller than the available mass, then N2 is the limiti ...
Interaction of Graphene Oxide with Proteins and
... strength of the buffer. The protein can be negatively (for instance carboxylate) or positively charged (for instance protonated amino groups), and the surface density of the oxygen-containing groups on the GO also varies with the preparation procedure and storage conditions. Based on this, various p ...
... strength of the buffer. The protein can be negatively (for instance carboxylate) or positively charged (for instance protonated amino groups), and the surface density of the oxygen-containing groups on the GO also varies with the preparation procedure and storage conditions. Based on this, various p ...
Lecture 4
... Proteins are flexible. One would like to align proteins modulo the flexibility. Hinge and shear protein domain motions (Gerstein, Lesk , Chotia). Conformational flexibility in drugs. ...
... Proteins are flexible. One would like to align proteins modulo the flexibility. Hinge and shear protein domain motions (Gerstein, Lesk , Chotia). Conformational flexibility in drugs. ...
Signs of Reaction - Calderglen High School
... Concentrated solutions have a large amount of solute in a relatively small volume of solvent. Dilute solutions have a relatively small amount of solute Calderglen High School ...
... Concentrated solutions have a large amount of solute in a relatively small volume of solvent. Dilute solutions have a relatively small amount of solute Calderglen High School ...
ME 2105 – Fall 2010 Suggested Homework Problems – The Key
... (a) The surface energy will be greater than the grain boundary energy. For grain boundaries, some atoms on one side of a boundary will bond to atoms on the other side; such is not the case for surface atoms. Therefore, there will be fewer unsatisfied bonds along a grain boundary. (b) The small-angle ...
... (a) The surface energy will be greater than the grain boundary energy. For grain boundaries, some atoms on one side of a boundary will bond to atoms on the other side; such is not the case for surface atoms. Therefore, there will be fewer unsatisfied bonds along a grain boundary. (b) The small-angle ...
Chapter 12 Stoichiometry - Ponder Independent School District
... you are given one dozen loaves of bread, a gallon of mustard, and three pieces of salami, how many salami sandwiches can you make? The limiting reagent is the reactant you run out of first. The excess reagent is the one you have left over. The limiting reagent determines how much product you c ...
... you are given one dozen loaves of bread, a gallon of mustard, and three pieces of salami, how many salami sandwiches can you make? The limiting reagent is the reactant you run out of first. The excess reagent is the one you have left over. The limiting reagent determines how much product you c ...
Document
... In order for a substance to move between the states of matter; for example, to turn from a solid into a liquid, which is called fusion, or from a liquid to a gas (vaporization), energy must be gained or lost. (As we move from solid to gas it is gained and from gas to a solid it is lost. Why? Molar H ...
... In order for a substance to move between the states of matter; for example, to turn from a solid into a liquid, which is called fusion, or from a liquid to a gas (vaporization), energy must be gained or lost. (As we move from solid to gas it is gained and from gas to a solid it is lost. Why? Molar H ...
Python Libraries for Computational Chemistry and Biology
... The primary model is a linear sequence of subunits drawn from a small pool of possible types (4 possible bases in DNA, 20 possible residues in protein), plus random mutation and evolution. ...
... The primary model is a linear sequence of subunits drawn from a small pool of possible types (4 possible bases in DNA, 20 possible residues in protein), plus random mutation and evolution. ...
2 - Ponder ISD
... you are given one dozen loaves of bread, a gallon of mustard, and three pieces of salami, how many salami sandwiches can you make? The limiting reagent is the reactant you run out of first. The excess reagent is the one you have left over. The limiting reagent determines how much product you c ...
... you are given one dozen loaves of bread, a gallon of mustard, and three pieces of salami, how many salami sandwiches can you make? The limiting reagent is the reactant you run out of first. The excess reagent is the one you have left over. The limiting reagent determines how much product you c ...
Chapter 5 ppt
... Products of a Reaction: Substances formed as a result of the reaction; written on the right side of the equation representing the reaction ...
... Products of a Reaction: Substances formed as a result of the reaction; written on the right side of the equation representing the reaction ...
Chemical Equations Chemical Reaction: Interaction between
... The arrow points towards the products formed by the reaction Individual products and reactants are separated by a plus sign Chemical Equation: A written statement using symbols and formulas to describe the changes that occur in a reaction Example: 2H2(g) + O2 (g) Æ 2H2O (l) Letter in parentheses ind ...
... The arrow points towards the products formed by the reaction Individual products and reactants are separated by a plus sign Chemical Equation: A written statement using symbols and formulas to describe the changes that occur in a reaction Example: 2H2(g) + O2 (g) Æ 2H2O (l) Letter in parentheses ind ...
Glossary - Chemistry (Intro)
... Notation of E.: Elements of the periodic table are assigned with a mass- and atomic number to quantify its number of protons (Z) and number of protons and neutrons (A); see chemistry atom. Representative E.: Elements in groups 1A through 7A, all of which have incompletely filled s or p subshell of h ...
... Notation of E.: Elements of the periodic table are assigned with a mass- and atomic number to quantify its number of protons (Z) and number of protons and neutrons (A); see chemistry atom. Representative E.: Elements in groups 1A through 7A, all of which have incompletely filled s or p subshell of h ...
Review Structural glycobiology: A game of snakes and ladders
... To help explore the concepts of the carbohydrate structure and recognition, let us compare carbohydrates to another flexible object, a snake. To the extent that a living snake is a flexible 3D object that is not random in its motional properties, it serves as a useful analogy for carbohydrate struct ...
... To help explore the concepts of the carbohydrate structure and recognition, let us compare carbohydrates to another flexible object, a snake. To the extent that a living snake is a flexible 3D object that is not random in its motional properties, it serves as a useful analogy for carbohydrate struct ...
KEY + + - UIC Department of Chemistry
... H2(g) 4. Identify the limiting reactant when 5.0 g of Fe react with 5.0 g of water. (a) Fe (b) H2 O 5. What mass of Fe2 O4 can be produced from the amounts of Fe and water in question 4? (a) 48.8 g (b) 49 g (c) 7.86 g (d) 7.9 g (e) 12 g 6. Given the answer in question 5, what is the actual yield of ...
... H2(g) 4. Identify the limiting reactant when 5.0 g of Fe react with 5.0 g of water. (a) Fe (b) H2 O 5. What mass of Fe2 O4 can be produced from the amounts of Fe and water in question 4? (a) 48.8 g (b) 49 g (c) 7.86 g (d) 7.9 g (e) 12 g 6. Given the answer in question 5, what is the actual yield of ...