Chemistry 400
... 8) Choose the transition (in a hydrogen atom) below that represents the absorption of the shortest wavelength photon. A) n = 1 to n = 2 B) n = 2 to n = 3 C) n = 4 to n = 5 D) n = 6 to n = 3 E) n = 3 to n = 1 9) Which of the following statements is TRUE? A) We can sometimes know the exact location an ...
... 8) Choose the transition (in a hydrogen atom) below that represents the absorption of the shortest wavelength photon. A) n = 1 to n = 2 B) n = 2 to n = 3 C) n = 4 to n = 5 D) n = 6 to n = 3 E) n = 3 to n = 1 9) Which of the following statements is TRUE? A) We can sometimes know the exact location an ...
SCHLOSS RINGBERG
... dynamical properties of solid surfaces [1]. Because of the low energies used (10-100 meV), monoenergetic beams of neutral He atoms probe the top most surface layer of any material in a completely nondestructive manner, and can be used equally well to investigate insulating or conducting materials. H ...
... dynamical properties of solid surfaces [1]. Because of the low energies used (10-100 meV), monoenergetic beams of neutral He atoms probe the top most surface layer of any material in a completely nondestructive manner, and can be used equally well to investigate insulating or conducting materials. H ...
Nawel BAGHDADLI, Françoise FIAT, Gustavo S
... SEM images (A/D) shows failure of scales, local lift and loss of cuticle edge. AFM images (B/E) (10µm x 10µm), shows damages for the same scale after treatment (“puffiness, bubbles”) and edge (broken). Where, TEM observations (C/F) of hair cuts shows degradations in the cortex area and important def ...
... SEM images (A/D) shows failure of scales, local lift and loss of cuticle edge. AFM images (B/E) (10µm x 10µm), shows damages for the same scale after treatment (“puffiness, bubbles”) and edge (broken). Where, TEM observations (C/F) of hair cuts shows degradations in the cortex area and important def ...
1-Structure of Heme
... pocket of the protein and the myoglobin-heme interaction is stabilized by hydrophobic attractions. • The heme group stabilizes the tertiary structure of ...
... pocket of the protein and the myoglobin-heme interaction is stabilized by hydrophobic attractions. • The heme group stabilizes the tertiary structure of ...
Chapter 4: Reactions in Aqueous Solution
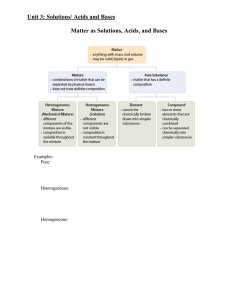
... 2) Many reactions take place in aqueous solution. The term aqueous means dissolved in water. 3) Hydration of solids in Water A) Solid dissolves (falls apart) through interaction of ions with water. B) Newly formed ions are now “FREE TO MOVE”. C) “Like Dissolves Like” i) polar materials dissolve in p ...
... 2) Many reactions take place in aqueous solution. The term aqueous means dissolved in water. 3) Hydration of solids in Water A) Solid dissolves (falls apart) through interaction of ions with water. B) Newly formed ions are now “FREE TO MOVE”. C) “Like Dissolves Like” i) polar materials dissolve in p ...
Attributes Tutorial
... If you have internet connectivity, structures can be obtained directly from the Protein Data Bank. Choose File... Fetch by ID from the Chimera menu and use the resulting dialog to fetch 1zik from the PDB. If you do not have internet connectivity, download the file 1zik.pdb included with this tutoria ...
... If you have internet connectivity, structures can be obtained directly from the Protein Data Bank. Choose File... Fetch by ID from the Chimera menu and use the resulting dialog to fetch 1zik from the PDB. If you do not have internet connectivity, download the file 1zik.pdb included with this tutoria ...
Slow Protein Conformational Change, Allostery and
... consideration of the biochemical/biophysical properties of each composing macromolecule. One of the reasons that account for the current state of affair is due to a lack of experimental data and theoretical understanding in the "intermediate regime" between single-molecule studies of individual enzy ...
... consideration of the biochemical/biophysical properties of each composing macromolecule. One of the reasons that account for the current state of affair is due to a lack of experimental data and theoretical understanding in the "intermediate regime" between single-molecule studies of individual enzy ...
Amino Acid Starter Kit©
... conditions discussed so far. a. First, have your students fold their proteins so that all of the hydrophobic sidechains are buried on the inside of their globular proteins, where they are hidden from polar water molecules. b. Then, the charged sidechains (Acidic and Basic) should be folded so that t ...
... conditions discussed so far. a. First, have your students fold their proteins so that all of the hydrophobic sidechains are buried on the inside of their globular proteins, where they are hidden from polar water molecules. b. Then, the charged sidechains (Acidic and Basic) should be folded so that t ...
Outline for Unit 1 Solutions, Acid/Base, and Gases
... 1. Nature of solute and solvent – like dissolves like 2. Agitation – solute dissolves more rapidly since fresh solvent is brought into contact with the solute. Only affects the rate not amount of solute that dissolves. 3. Temperature – at higher temp kinetic energy of the solvent is higher so more c ...
... 1. Nature of solute and solvent – like dissolves like 2. Agitation – solute dissolves more rapidly since fresh solvent is brought into contact with the solute. Only affects the rate not amount of solute that dissolves. 3. Temperature – at higher temp kinetic energy of the solvent is higher so more c ...
- Information Extraction and Text Mining Group
... Motivation • assisting in the construction and updating of databases • providing structured summaries for queries What is known about protein X (subcellular & tissue localization, associations with diseases, interactions with drugs, …)? • assisting scientific discovery by detecting previously unkno ...
... Motivation • assisting in the construction and updating of databases • providing structured summaries for queries What is known about protein X (subcellular & tissue localization, associations with diseases, interactions with drugs, …)? • assisting scientific discovery by detecting previously unkno ...
Final Exam 4
... This exam is composed of 50 questions, 14 of which require mathematics that require a calculator. Go initially through the exam and answer the questions you can answer quickly. Then go back and try the ones that are more challenging to you and/or that require calculations. As discussed in the course ...
... This exam is composed of 50 questions, 14 of which require mathematics that require a calculator. Go initially through the exam and answer the questions you can answer quickly. Then go back and try the ones that are more challenging to you and/or that require calculations. As discussed in the course ...
Preface from the Textbook - McGraw Hill Higher Education
... stoichiometry and reaction classes, show how gas behavior is modeled, and highlight the relation between heat and chemical change. • Chapters 7 through 15 take an “atoms-first” approach, as they move from atomic structure and electron configuration to how atoms bond and what the resulting molecules ...
... stoichiometry and reaction classes, show how gas behavior is modeled, and highlight the relation between heat and chemical change. • Chapters 7 through 15 take an “atoms-first” approach, as they move from atomic structure and electron configuration to how atoms bond and what the resulting molecules ...
Page 1
... 54. State the Law of Conservation of Mass. States that mass is neither created nor destroyed in any process but is conserved 55. Compare covalent and ionic bonding. Covalent: results from the sharing of valence electrons; between 2 non-metals Ionic: electrostatic force that holds oppositely charged ...
... 54. State the Law of Conservation of Mass. States that mass is neither created nor destroyed in any process but is conserved 55. Compare covalent and ionic bonding. Covalent: results from the sharing of valence electrons; between 2 non-metals Ionic: electrostatic force that holds oppositely charged ...
Amino Acids
... β-Bends reverse the direction of a polyp chain, helping it form a compact, globular shape Usually found on surface of protein molecules & often include charged residues Generally composed of 4 aa’s, one of which may be proline. Gly is also frequently found in β-Bends. Stabilized by H-bonds and ionic ...
... β-Bends reverse the direction of a polyp chain, helping it form a compact, globular shape Usually found on surface of protein molecules & often include charged residues Generally composed of 4 aa’s, one of which may be proline. Gly is also frequently found in β-Bends. Stabilized by H-bonds and ionic ...
Fermionic phase-space method for exact quantum dynamics
... * Large deviations from mean-field methods (PMFT) for some correlations. * Justify PMFT for atom numbers and densities if the molecular depletion is small. * Diffusion gauges change the numerical performance and can qualitatively change the behaviour of ...
... * Large deviations from mean-field methods (PMFT) for some correlations. * Justify PMFT for atom numbers and densities if the molecular depletion is small. * Diffusion gauges change the numerical performance and can qualitatively change the behaviour of ...
Sodium dodecyl sulfate (L3771)
... Sigma-Aldrich, Inc. warrants that its products conform to the information contained in this and other Sigma-Aldrich publications. Purchaser must determine the suitability of the product(s) for their particular use. Additional terms and conditions may apply. Please see reverse side of the invoice or ...
... Sigma-Aldrich, Inc. warrants that its products conform to the information contained in this and other Sigma-Aldrich publications. Purchaser must determine the suitability of the product(s) for their particular use. Additional terms and conditions may apply. Please see reverse side of the invoice or ...
Periodic Table and the Atom Answers
... c) At a smaller volume the atoms will have less room to move around, so they will collide with the sides more often. d) The initial statement is false. Gas pressures do not increase when the volume is decreased. 6) What are the five assumptions we make about an ideal gas? ...
... c) At a smaller volume the atoms will have less room to move around, so they will collide with the sides more often. d) The initial statement is false. Gas pressures do not increase when the volume is decreased. 6) What are the five assumptions we make about an ideal gas? ...
Chapter 3 Stoichiometry: Calculations with Chemical Formulas and
... In theory we could have a mole of whatever thing!! 1 mole of paper clips = 6.022*1023 paper clips 1 mole of tortillas = 6.022*1023 tortillas 1 mole of cars =6.022*1023 cars 1 mole of carbon atoms = 6.022*1023 C atoms 1 mole of H2O = 6.022*1023 H2O molecules 1 mole of NaCl = 6.022*1023 NaCl formula u ...
... In theory we could have a mole of whatever thing!! 1 mole of paper clips = 6.022*1023 paper clips 1 mole of tortillas = 6.022*1023 tortillas 1 mole of cars =6.022*1023 cars 1 mole of carbon atoms = 6.022*1023 C atoms 1 mole of H2O = 6.022*1023 H2O molecules 1 mole of NaCl = 6.022*1023 NaCl formula u ...
The in vitro catalysis of protein folding by endoplasmic reticulum
... prolyl peptide bonds (4). Isomerisations of Xaa-Pro peptide bonds have been identified as slow steps in in v i m folding of some proteins ( 5 ) . Nascent proteins are presumably all fruns polypeptide chains and cis Xaa-Pro peptide bonds are common under native conditions (6); this suggests a role fo ...
... prolyl peptide bonds (4). Isomerisations of Xaa-Pro peptide bonds have been identified as slow steps in in v i m folding of some proteins ( 5 ) . Nascent proteins are presumably all fruns polypeptide chains and cis Xaa-Pro peptide bonds are common under native conditions (6); this suggests a role fo ...
Molecular Docking
... Molecular mechanics can be used to estimate the internal energy of the system, which makes it useful for calculating ΔG The total energy of a system is described as the sum of the independent terms of the force field The energies obtained by force field methods can be used directly to approximat ...
... Molecular mechanics can be used to estimate the internal energy of the system, which makes it useful for calculating ΔG The total energy of a system is described as the sum of the independent terms of the force field The energies obtained by force field methods can be used directly to approximat ...
Protein_structure_II
... more than 1.8 million non-redundant protein sequences in UniProt (Swiss-Prot + TrEMBL). • Computational structure prediction may provide valuable information for most of the protein sequences derived from genome sequencing projects. • Three predictive methods: – Homology (or comparative) modeling. ...
... more than 1.8 million non-redundant protein sequences in UniProt (Swiss-Prot + TrEMBL). • Computational structure prediction may provide valuable information for most of the protein sequences derived from genome sequencing projects. • Three predictive methods: – Homology (or comparative) modeling. ...
How are proteins broken down?
... such as disaccharides (two linked simple sugars) and polysaccharides (many linked simple sugars). Complex sugars must be broken down into simple sugars before your body can use them. ...
... such as disaccharides (two linked simple sugars) and polysaccharides (many linked simple sugars). Complex sugars must be broken down into simple sugars before your body can use them. ...
Ab initio modelling tutorial (part II)
... multiple models as criteria for grouping – Normalized spatial deviation serves as a distance between heterogeneous models (e.g. bead models) – R.m.s.d. is employed for those with atom-to-atom correspondence (e.g. rigid body models) ...
... multiple models as criteria for grouping – Normalized spatial deviation serves as a distance between heterogeneous models (e.g. bead models) – R.m.s.d. is employed for those with atom-to-atom correspondence (e.g. rigid body models) ...
Chapter 4B Lecture
... conformational entropy and relatively high free energy. As folding proceeds, the narrowing of the funnel reflects the decrease in the conformational space that must be searched as the protein approaches its native state (N, at the bottom of the funnel). Small depressions along the sides of the free ...
... conformational entropy and relatively high free energy. As folding proceeds, the narrowing of the funnel reflects the decrease in the conformational space that must be searched as the protein approaches its native state (N, at the bottom of the funnel). Small depressions along the sides of the free ...
Chem 2A Final Review
... 60. The oxidation number (oxidation state) of sulfur in the following are? K2SO2, K2S2O3, K2S 61. According to Le Chatelier’s principle what effects will take place on the equilibrium of the following reaction: CO2 + H2 H2O + CO a) Increase [H2] b) Increase [H2O] c) remove H2O and CO ...
... 60. The oxidation number (oxidation state) of sulfur in the following are? K2SO2, K2S2O3, K2S 61. According to Le Chatelier’s principle what effects will take place on the equilibrium of the following reaction: CO2 + H2 H2O + CO a) Increase [H2] b) Increase [H2O] c) remove H2O and CO ...