Complexation - International Islamic University Malaysia
... When oxygen gets to where it is needed, it breaks away from haemoglobin, which returns to the lungs to get some more oxygen. • carbon monoxide is poisonous and it reacts with ...
... When oxygen gets to where it is needed, it breaks away from haemoglobin, which returns to the lungs to get some more oxygen. • carbon monoxide is poisonous and it reacts with ...
Chapter 2 - U of L Class Index
... These complexes are also hydrates. (Recall Chemistry 1000!) Thus, a co-ordination complex must contain a transition metal cation and several ligands. It may also have counter ion(s) (to balance charge) or extra water molecules. When naming a coordination complex or complex salt, look for these compo ...
... These complexes are also hydrates. (Recall Chemistry 1000!) Thus, a co-ordination complex must contain a transition metal cation and several ligands. It may also have counter ion(s) (to balance charge) or extra water molecules. When naming a coordination complex or complex salt, look for these compo ...
Lewis Acids and Bases - Screenshot for timg.co.il
... There are reactions in nonaqueous solvents, in the gaseous state, and even in the solid state that can be considered acid–base reactions in which Brønsted–Lowry theory is not adequate to explain. ...
... There are reactions in nonaqueous solvents, in the gaseous state, and even in the solid state that can be considered acid–base reactions in which Brønsted–Lowry theory is not adequate to explain. ...
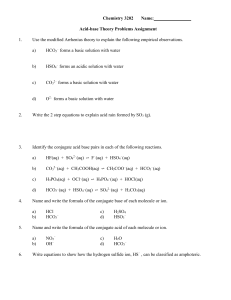
Chemistry 3202 Name: Acid-base Theory Problems Assignment 1
... Strong acids, such as perchloric acid, have been shown to react quantitatively with strong bases, such as sodium hydroxide. ...
... Strong acids, such as perchloric acid, have been shown to react quantitatively with strong bases, such as sodium hydroxide. ...
Document
... added to NH3 solution, an intense purple solution forms. This indicates the probable formation of a complex ion. Complex ions can be isolated as salts. In this case, the sulfate salt of the copper(II)/ammonia complex is soluble in water but insoluble in ethanol. It is therefore convenient to isolate ...
... added to NH3 solution, an intense purple solution forms. This indicates the probable formation of a complex ion. Complex ions can be isolated as salts. In this case, the sulfate salt of the copper(II)/ammonia complex is soluble in water but insoluble in ethanol. It is therefore convenient to isolate ...
5.3.1 Transition Elements 2012
... directions. The central ion is not described as chiral; this term is reserved for organic molecules only. On the next page is a diagram showing the two isomers from a complex with three ethanediamine (en) ligands around an M+ metal ion ...
... directions. The central ion is not described as chiral; this term is reserved for organic molecules only. On the next page is a diagram showing the two isomers from a complex with three ethanediamine (en) ligands around an M+ metal ion ...
Exercises for Advanced Inorganic Chemistry, Part Coordination
... with two unpaired electrons. Likewise, [Fe(CN)6]3- has only one unpaired electron but [Fe(H2O)6]3+ has five. Explain these observations using a) valence bond theory and b) simple crystal field theory. ...
... with two unpaired electrons. Likewise, [Fe(CN)6]3- has only one unpaired electron but [Fe(H2O)6]3+ has five. Explain these observations using a) valence bond theory and b) simple crystal field theory. ...
Chapter 14, Section 1, pages 494-501
... A chemical equilibrium is a state of balance between the forward and reverse reactions. The concentration of products and reactants remains unchanged. H2 + I2 <--------------> 2HI See fig. 3, pg. 498 Chemical Equilibria Are Dynamic (Leaky boat) Demo-pg. 500 Static equilibrium is a state when nothing ...
... A chemical equilibrium is a state of balance between the forward and reverse reactions. The concentration of products and reactants remains unchanged. H2 + I2 <--------------> 2HI See fig. 3, pg. 498 Chemical Equilibria Are Dynamic (Leaky boat) Demo-pg. 500 Static equilibrium is a state when nothing ...
Chem 4B First Midterm Review Sheet
... Chelate effect- the observation that a single multidentate ligand forms metal complexes that are more stable than those formed by several individual ligands with the same ligand atoms Complexometric titration- one in which the reaction between analyte and titrant involves complexes formation Formati ...
... Chelate effect- the observation that a single multidentate ligand forms metal complexes that are more stable than those formed by several individual ligands with the same ligand atoms Complexometric titration- one in which the reaction between analyte and titrant involves complexes formation Formati ...
Answer - Assignment Expert
... A complex is a substance in which a metal atom or ion is associated with a group of neutral molecules or anions called ligands. Coordination compounds are neutral substances (i.e. uncharged) in which at least one ion is present as a complex. The coordination compounds are named in the following way. ...
... A complex is a substance in which a metal atom or ion is associated with a group of neutral molecules or anions called ligands. Coordination compounds are neutral substances (i.e. uncharged) in which at least one ion is present as a complex. The coordination compounds are named in the following way. ...
Chapter 1 Structure and Bonding
... Factors affecting the geometry of a coordination compound 1) Prediction can be difficult 2) VSEPR usually is a good first approximation; don’t count the d-electrons 3) Maximize the number of bonds (more bonds = more stable) 4) Occupancy of the d-orbitals (Chapter 10) 5) Steric interference by large ...
... Factors affecting the geometry of a coordination compound 1) Prediction can be difficult 2) VSEPR usually is a good first approximation; don’t count the d-electrons 3) Maximize the number of bonds (more bonds = more stable) 4) Occupancy of the d-orbitals (Chapter 10) 5) Steric interference by large ...
chapter15
... • Most transition metal complexes are brightly colored • Exception: those with empty d sublevels (e.g., Sc3+; those with full d sublevels (e.g., Zn2+) • The splitting of the d sublevel results in an energy difference that corresponds to the visible region of the electromagnetic spectrum • Visible li ...
... • Most transition metal complexes are brightly colored • Exception: those with empty d sublevels (e.g., Sc3+; those with full d sublevels (e.g., Zn2+) • The splitting of the d sublevel results in an energy difference that corresponds to the visible region of the electromagnetic spectrum • Visible li ...
Irradiations of the transition metal-to
... of a series of cyanide-bridged chromium(III)−ruthenium(II) complexes at 77 K leads to nearinfrared emission spectra of the corresponding chromium(II)−ruthenium(III) electron transfer excited states. The lifetimes of most of the MMCT excited states increase more than 10-fold when their am(m)ine ligan ...
... of a series of cyanide-bridged chromium(III)−ruthenium(II) complexes at 77 K leads to nearinfrared emission spectra of the corresponding chromium(II)−ruthenium(III) electron transfer excited states. The lifetimes of most of the MMCT excited states increase more than 10-fold when their am(m)ine ligan ...
Lecture 3 - Classification and Nomenclature
... For a given metal ion, the thermodynamic stability of a chelated complex involving bidentate or polydentate ligands is greater than that of a complex containing a corresponding number of comparable monodentate ligands. This is called the chelate effect. 5-membered rings are more stable than 6, 4-mem ...
... For a given metal ion, the thermodynamic stability of a chelated complex involving bidentate or polydentate ligands is greater than that of a complex containing a corresponding number of comparable monodentate ligands. This is called the chelate effect. 5-membered rings are more stable than 6, 4-mem ...