Complexes of Ethylenediaminetetracarboxylate Ligands
... increasing the size or rigidity of the chains or omitting one or more of the carboxylate arms. Geometrical isomerism is possible for complexes of the hexadentate edta-type ligands where the carboxylate arms are replaced so as to form nonequivalent chelate rings.9,10 Structural parameters of the [M(e ...
... increasing the size or rigidity of the chains or omitting one or more of the carboxylate arms. Geometrical isomerism is possible for complexes of the hexadentate edta-type ligands where the carboxylate arms are replaced so as to form nonequivalent chelate rings.9,10 Structural parameters of the [M(e ...
type worksheet title here
... 7) What is the molar concentration of ethanol in a solution made by mixing 23 g of ethanol with 200 mL of water? (density of ethanol = 0.789 g/mL and MW ethanol = 46 g/mol) ...
... 7) What is the molar concentration of ethanol in a solution made by mixing 23 g of ethanol with 200 mL of water? (density of ethanol = 0.789 g/mL and MW ethanol = 46 g/mol) ...
The Representative Elements
... sublevel. Because these inner orbitals cannot participate as easily in bonding as the s and p orbitals the chemistry is not greatly affected by the gradual change in the increased number of electrons in the d orbital. ...
... sublevel. Because these inner orbitals cannot participate as easily in bonding as the s and p orbitals the chemistry is not greatly affected by the gradual change in the increased number of electrons in the d orbital. ...
The Representative Elements
... sublevel. Because these inner orbitals cannot participate as easily in bonding as the s and p orbitals the chemistry is not greatly affected by the gradual change in the increased number of electrons in the d orbital. ...
... sublevel. Because these inner orbitals cannot participate as easily in bonding as the s and p orbitals the chemistry is not greatly affected by the gradual change in the increased number of electrons in the d orbital. ...
Chemistry Homework Help - Tutor
... Transition metals are found in nature Rocks and minerals contain transition metals The color of many gemstones is due to the presence of transition metal ions ...
... Transition metals are found in nature Rocks and minerals contain transition metals The color of many gemstones is due to the presence of transition metal ions ...
Document
... 273.16 deg ree.mole 0.082057 litre.atm / deg ree.mole PV RT R 0.082057 litres.atm / deg ree.mole 8.3144 joules / deg ree.mole ...
... 273.16 deg ree.mole 0.082057 litre.atm / deg ree.mole PV RT R 0.082057 litres.atm / deg ree.mole 8.3144 joules / deg ree.mole ...
A Problem with Spin
... The low-spin complexes, e.g. [Mn(CN)6]4- and [Mn(CNR)6]4- (R=CH3, C2H5), have been studied much less frequently than the high-spin complexes because their occurrence in biological and other systems is very rare. In the present work we intended to determine the spin-states of several manganese comple ...
... The low-spin complexes, e.g. [Mn(CN)6]4- and [Mn(CNR)6]4- (R=CH3, C2H5), have been studied much less frequently than the high-spin complexes because their occurrence in biological and other systems is very rare. In the present work we intended to determine the spin-states of several manganese comple ...
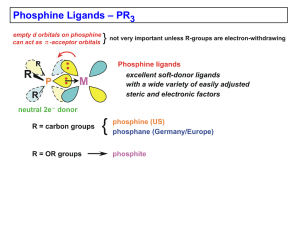
Chapter 4 (Phosphines)
... • the alkyl phosphines are strong s-donors; low MW ones usually colorless liquids, somewhat to very air-sensitive, horrible smelling (unless very high MW and nonvolatile) Poor s-Donors, Good p-Acceptors: Phosphites: P(OMe)3 (107°), P(OEt)3 (110°), P(OPh)3 (128°) • phosphites are relatively poor s-do ...
... • the alkyl phosphines are strong s-donors; low MW ones usually colorless liquids, somewhat to very air-sensitive, horrible smelling (unless very high MW and nonvolatile) Poor s-Donors, Good p-Acceptors: Phosphites: P(OMe)3 (107°), P(OEt)3 (110°), P(OPh)3 (128°) • phosphites are relatively poor s-do ...
- White Rose Research Online
... adduct {Zn[Ru(ttpy)(CN)3]}+ in which, presumably, those coordination sites around the Zn2+ ion that are not occupied by cyanide ligands are occupied by MeCN solvent molecules, water molecules [since the Zn(ClO4)2 is hydrated] and/or perchlorate anions. The calculated best fit (as used in Figure 5) f ...
... adduct {Zn[Ru(ttpy)(CN)3]}+ in which, presumably, those coordination sites around the Zn2+ ion that are not occupied by cyanide ligands are occupied by MeCN solvent molecules, water molecules [since the Zn(ClO4)2 is hydrated] and/or perchlorate anions. The calculated best fit (as used in Figure 5) f ...
South Pasadena • AP Chemistry
... 26. How many grams of Na2CO3 (molar mass = 106.0 g/mol) are required for complete reaction with 25.0 mL of 0.155 M HNO3? Na2CO3 + 2HNO3 2NaNO3 + CO2 + H2O a) 0.122 g b) 0.205 g c) 0.410 g ...
... 26. How many grams of Na2CO3 (molar mass = 106.0 g/mol) are required for complete reaction with 25.0 mL of 0.155 M HNO3? Na2CO3 + 2HNO3 2NaNO3 + CO2 + H2O a) 0.122 g b) 0.205 g c) 0.410 g ...
Chapter 4 - Aqueous Reactions
... Note that the equation is balanced for both mass and charge!!! ...
... Note that the equation is balanced for both mass and charge!!! ...
Le Chatelier`s Principle Quiz Answer Key
... 6. The production of ammonia from ammonium chloride will increase at higher temperature. 7. In the above reaction system, the value of Keq decreases as the temperature increases. 8. For the above reaction at equilibrium, an increase in pressure on the system causes a decrease in gaseous ammonia conc ...
... 6. The production of ammonia from ammonium chloride will increase at higher temperature. 7. In the above reaction system, the value of Keq decreases as the temperature increases. 8. For the above reaction at equilibrium, an increase in pressure on the system causes a decrease in gaseous ammonia conc ...
Coordination properties of the diethyl 2-quinolilmethylphosphonate ligand with chloride
... bonded quinoline group, the characteristic ligand band assigned to the P=O group remains almost at the same position as in the free ligand, indicating monodentate coordination of 2-qmpe. In fact, X-ray structural determination [25] of trans-[Pd(2qmpe)2Cl2] confirmed that the metal ion is bound in a ...
... bonded quinoline group, the characteristic ligand band assigned to the P=O group remains almost at the same position as in the free ligand, indicating monodentate coordination of 2-qmpe. In fact, X-ray structural determination [25] of trans-[Pd(2qmpe)2Cl2] confirmed that the metal ion is bound in a ...
Ch 19 test_take-home
... △G° at 298 oK for this reaction is -33.3 kJ/mol. What is the value of △G at 298 K for a reaction mixture that consists of 1.9 atm N2, 1.6 atm H2, and 0.65 atm NH3? ...
... △G° at 298 oK for this reaction is -33.3 kJ/mol. What is the value of △G at 298 K for a reaction mixture that consists of 1.9 atm N2, 1.6 atm H2, and 0.65 atm NH3? ...
AND BINUCLEAR COMPLEXES CONTAINING OXIME LIGANDS
... Spectrum-100 Perkin Elmer spectrometer in the range of 400-4000 cm-1. X-ray data were collected at room temperature on an Oxford Diffraction Xcalibur diffractometer equipped with CCD area detector and a graphite monochromator utilizing MoKα radiation. Final unit cell dimensions were obtained and ref ...
... Spectrum-100 Perkin Elmer spectrometer in the range of 400-4000 cm-1. X-ray data were collected at room temperature on an Oxford Diffraction Xcalibur diffractometer equipped with CCD area detector and a graphite monochromator utilizing MoKα radiation. Final unit cell dimensions were obtained and ref ...