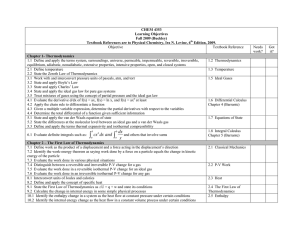
Organometallic MT Complexes
... MT Carbonyls The CO stretching frequency is often used to determine the structure of these compounds. The carbon monoxide molecule can be terminal, or bridge between 2 or 3 metal atoms. The CO stretching frequency decreases with increased bonding to metals. As the π* orbital on CO receives electron ...
... MT Carbonyls The CO stretching frequency is often used to determine the structure of these compounds. The carbon monoxide molecule can be terminal, or bridge between 2 or 3 metal atoms. The CO stretching frequency decreases with increased bonding to metals. As the π* orbital on CO receives electron ...
Ap Chem Seminar Notes
... acidic solution. If the solution is then titrated with NaOH, at what pH will the indicator color change first be visible? (p. 753 Zum) ...
... acidic solution. If the solution is then titrated with NaOH, at what pH will the indicator color change first be visible? (p. 753 Zum) ...
Feasibility Study of using FAIMS to Detect Carbonyl Sulfide in Propane
... Sample preparation and introduction FAIMS can be used to detect volatiles in aqueous, solid and gaseous matrices and can consequently be used for a wide variety of applications. The user requirements and sample matrix for each application define the sample preparation and introduction steps required ...
... Sample preparation and introduction FAIMS can be used to detect volatiles in aqueous, solid and gaseous matrices and can consequently be used for a wide variety of applications. The user requirements and sample matrix for each application define the sample preparation and introduction steps required ...
Effects of Gamma Radiation on Iron (III) Complex with Sodium
... Iron- salicylate complex has been studied. The structure of the iron- salicylate complex has been studied. It was found that the molar ratio of the formed complex determined was 1: 1.5. The color bleaching of the complex solutions upon irradiation was followed spectrophotometrically and the % color ...
... Iron- salicylate complex has been studied. The structure of the iron- salicylate complex has been studied. It was found that the molar ratio of the formed complex determined was 1: 1.5. The color bleaching of the complex solutions upon irradiation was followed spectrophotometrically and the % color ...
1.Isomerism Notes
... isomerism and optical isomerism. These are discussed below. 1. Geometrical Isomerism. Geometrical isomerism is due to ligands occupying different positions around the central ion. The ligands occupy positions either adjacent to one another or opposite to one another. These are referred to as cls for ...
... isomerism and optical isomerism. These are discussed below. 1. Geometrical Isomerism. Geometrical isomerism is due to ligands occupying different positions around the central ion. The ligands occupy positions either adjacent to one another or opposite to one another. These are referred to as cls for ...
coordination compounds - Ahlcon Public School , Mayur Vihar Ph
... a) CO3+ ion is bound to one Cl-, one NH3 molecule and two bidentate ethylene diamine molecules. b) Ni2+ ion is bound to two water molecules and two oxalate ions. CBSE – 2011 Write the name and magnetic behavior of each of the above coordination entities. (At. No CO – 27 Ni – 28) CBSE – 2011 23. A co ...
... a) CO3+ ion is bound to one Cl-, one NH3 molecule and two bidentate ethylene diamine molecules. b) Ni2+ ion is bound to two water molecules and two oxalate ions. CBSE – 2011 Write the name and magnetic behavior of each of the above coordination entities. (At. No CO – 27 Ni – 28) CBSE – 2011 23. A co ...
Ch. 16
... ΔSº reaction Sº products Sº reactants - generally, the more complex the molecule, the higher the standard entropy (more rotational and ...
... ΔSº reaction Sº products Sº reactants - generally, the more complex the molecule, the higher the standard entropy (more rotational and ...
Net Ionic Prep Session NMSI INSTRUCTOR
... WRITE THE REACTANT SETS FOR ALL THREE. Spend 3 minutes writing products using the solubility rules and strong acidbase guidelines listed on the other side of this page. To get the easy three points involved with step SIX above do the following: Write the reactants. On the product side, open a set of ...
... WRITE THE REACTANT SETS FOR ALL THREE. Spend 3 minutes writing products using the solubility rules and strong acidbase guidelines listed on the other side of this page. To get the easy three points involved with step SIX above do the following: Write the reactants. On the product side, open a set of ...
Co(NH 3 ) 5 (NO 2 ) - Department of Chemistry
... the bacteria. It was approved for cancer treatment in 1978. ...
... the bacteria. It was approved for cancer treatment in 1978. ...
Preparation and structural comparison of the ruthenium (0
... kinetics with koW = 1.5 X l C 7 s-l. Bimolecular substitution would have been revealed by curvature of the In (% [RU(DMPE)~(nap)H]) vs. time plot after 1-2 half-lives, but no such curvature was found. There was no evidence for formation of Ru(DMPE),(C6D5)D during the reaction. Compound 1 reacts with ...
... kinetics with koW = 1.5 X l C 7 s-l. Bimolecular substitution would have been revealed by curvature of the In (% [RU(DMPE)~(nap)H]) vs. time plot after 1-2 half-lives, but no such curvature was found. There was no evidence for formation of Ru(DMPE),(C6D5)D during the reaction. Compound 1 reacts with ...
Table 2
... atom in the compound [Co(NH3)5Cl]Cl2? 3- Coordination compounds which contain cyanide, CN-, ligands tend to be yellow whereas coordination compounds which contain water, H2O, ligands tend to be blue or. Explain why? 4-Consider the coordination compound, Na3[Co(CN)6]. Would you expect this compound t ...
... atom in the compound [Co(NH3)5Cl]Cl2? 3- Coordination compounds which contain cyanide, CN-, ligands tend to be yellow whereas coordination compounds which contain water, H2O, ligands tend to be blue or. Explain why? 4-Consider the coordination compound, Na3[Co(CN)6]. Would you expect this compound t ...
Chapter 4. Hard and Soft Acid/Base Theory based on Lewis Acids
... from base to acid results in the joining of the acid and base via a covalent bond. The bonded acid-base species is called an adduct (short for addition product), a coordination compound, or a complex. Lewis theory puts the emphasis on the donation and acceptance of electrons; this is appropriate, be ...
... from base to acid results in the joining of the acid and base via a covalent bond. The bonded acid-base species is called an adduct (short for addition product), a coordination compound, or a complex. Lewis theory puts the emphasis on the donation and acceptance of electrons; this is appropriate, be ...
Equilibrium
... According to the collision theory, atoms, ions, and molecules can react to form products when they collide with one another, provided that the colliding particles have enough kinetic energy. Particles lacking the necessary kinetic energy to react, bounce apart unchanged when they collide. To illustr ...
... According to the collision theory, atoms, ions, and molecules can react to form products when they collide with one another, provided that the colliding particles have enough kinetic energy. Particles lacking the necessary kinetic energy to react, bounce apart unchanged when they collide. To illustr ...
Chemical Equilibrium
... slightly soluble in water and equations are written to represent the equilibrium between the compound and the ions present in a saturated aqueous solution. • The solubility product constant, Ksp, is the product of the concentrations of the ions involved in a solubility equilibrium, each raised to a ...
... slightly soluble in water and equations are written to represent the equilibrium between the compound and the ions present in a saturated aqueous solution. • The solubility product constant, Ksp, is the product of the concentrations of the ions involved in a solubility equilibrium, each raised to a ...
H 3 O - TeacherWeb
... Calculate the pH of a 0.30 M solution of acetic acid, HC2H3O2, at 25C. HC2H3O2 (aq) + H2O (l) ...
... Calculate the pH of a 0.30 M solution of acetic acid, HC2H3O2, at 25C. HC2H3O2 (aq) + H2O (l) ...
2012 Chem 13 News Exam
... Given the thermochemical equations above, what is the standard enthalpy change for the reaction below? The answers are expressed in kJ per mole of C2H6. ...
... Given the thermochemical equations above, what is the standard enthalpy change for the reaction below? The answers are expressed in kJ per mole of C2H6. ...