Powerpoints - Holy Cross Collegiate
... • In these cases, the amount of product that results from a chemical reaction is limited by the reactant that is used up or completely consumed first. • The reactant that is completely used up in the reaction is called the limiting reactant. It is also known as the limiting reagent. • Any reactant(s ...
... • In these cases, the amount of product that results from a chemical reaction is limited by the reactant that is used up or completely consumed first. • The reactant that is completely used up in the reaction is called the limiting reactant. It is also known as the limiting reagent. • Any reactant(s ...
Acids and bases
... Levelling and differentiating effects Non-aqueous solvents that are good proton acceptors (e.g.NH3) encourage acids to ionize in them. In a basic solvent, all acids are strong. The solvent is said to exhibit a levelling effect on the acid, since the strength of the dissolved acid cannot excee ...
... Levelling and differentiating effects Non-aqueous solvents that are good proton acceptors (e.g.NH3) encourage acids to ionize in them. In a basic solvent, all acids are strong. The solvent is said to exhibit a levelling effect on the acid, since the strength of the dissolved acid cannot excee ...
Preparation of Mn(acac)
... Manganese is a first row transition metal that has a tremendous variety of oxidation states that appear in its compounds. The oxidation numbers range from Mn(–III) in compounds like Mn(NO)3CO to Mn(VII) in KMnO4. Compounds of manganese range in oxidation number between theses two extremes. This expe ...
... Manganese is a first row transition metal that has a tremendous variety of oxidation states that appear in its compounds. The oxidation numbers range from Mn(–III) in compounds like Mn(NO)3CO to Mn(VII) in KMnO4. Compounds of manganese range in oxidation number between theses two extremes. This expe ...
A density functional theory protocol for the calculation of redox
... and thermal corrections made to the Gibbs free energy at temperature T. The complexity in using this procedure to calculate redox potential comes from the fact that the inner-sphere coordination number of the Cu(I)/Cu(II) complexes is not always conserved during solvation. On the one hand, it is diffi ...
... and thermal corrections made to the Gibbs free energy at temperature T. The complexity in using this procedure to calculate redox potential comes from the fact that the inner-sphere coordination number of the Cu(I)/Cu(II) complexes is not always conserved during solvation. On the one hand, it is diffi ...
Formula - SandersScienceStuff
... of atoms that have a – charge. They are EASY to deal with in naming and formula writing if you think of them as ONE UNIT that can not be altered in any ...
... of atoms that have a – charge. They are EASY to deal with in naming and formula writing if you think of them as ONE UNIT that can not be altered in any ...
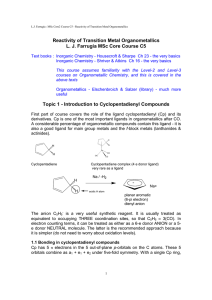
π bonded ligands (# 2)
... Most of the neutral ligands we have studied (apart from carbenes) have been stable in the free state. Cyclobutadienes on the other hand are highyl reactive when not complexed to a y g y p late transition metal. The free molecule, with four π electrons, is antiaromatic and rectangular, but the lig ...
... Most of the neutral ligands we have studied (apart from carbenes) have been stable in the free state. Cyclobutadienes on the other hand are highyl reactive when not complexed to a y g y p late transition metal. The free molecule, with four π electrons, is antiaromatic and rectangular, but the lig ...
AP® Chemistry
... 7. List the six strong acids. (HC) 8. Recognize Lewis acid-base reactions. Weak Ionic Equilibrium (2 ½ weeks) Chapter 15 I. Weak acids and bases A. pH B. pOH C. Buffer systems D. Hydrolysis II. Solubility Product A. Factors involving dissolution B. Molar solubility The student will: 1. Identify weak ...
... 7. List the six strong acids. (HC) 8. Recognize Lewis acid-base reactions. Weak Ionic Equilibrium (2 ½ weeks) Chapter 15 I. Weak acids and bases A. pH B. pOH C. Buffer systems D. Hydrolysis II. Solubility Product A. Factors involving dissolution B. Molar solubility The student will: 1. Identify weak ...
1 1. This question is about shapes of molecules
... iii) Reduction of A on a thermogravimetric analyser at 200 °C in hydrogen reduces all the copper 3+ in the material to copper 2+ and produces compound B. All the other elements remain in the same oxidation state. Given that the starting mass was 84.2 mg, what would be the mass of the remaining compo ...
... iii) Reduction of A on a thermogravimetric analyser at 200 °C in hydrogen reduces all the copper 3+ in the material to copper 2+ and produces compound B. All the other elements remain in the same oxidation state. Given that the starting mass was 84.2 mg, what would be the mass of the remaining compo ...
Equilibrium STUDY GUIDE by Keshara Senanayake ---
... All of this is factored back into solubility (which is the maximum quantity of that substance that call dissolve in a given solvent) at some point no additional AgCl can dissolve because the solution is saturated. Although ions continue to dissolve other ions begin to precipitate out of the solution ...
... All of this is factored back into solubility (which is the maximum quantity of that substance that call dissolve in a given solvent) at some point no additional AgCl can dissolve because the solution is saturated. Although ions continue to dissolve other ions begin to precipitate out of the solution ...
Two-Electron Reduction of a Vanadium(V) Nitride by CO to Release
... 150.6(1)°, on par with other isocyanate complexes.6 With 1-V(NCO) in hand, attention turned to probing the redox chemistry of the complex. When treated with 0.5% Na/Hg in the same manner as had been done for 1-Nb(NCO), 1-V(NCO) undergoes cyanate dissociation to give 1-V and sodium cyanate (Scheme 1B ...
... 150.6(1)°, on par with other isocyanate complexes.6 With 1-V(NCO) in hand, attention turned to probing the redox chemistry of the complex. When treated with 0.5% Na/Hg in the same manner as had been done for 1-Nb(NCO), 1-V(NCO) undergoes cyanate dissociation to give 1-V and sodium cyanate (Scheme 1B ...
The Thomas Hardye School Summer Preparation Task Chemistry AS
... Hydroxide Nitrate Oxide Sulphide Sulphate Carbonate Phosphate ...
... Hydroxide Nitrate Oxide Sulphide Sulphate Carbonate Phosphate ...
Franke_reactivity_of_uranium(IV)
... chalcogens, are considered to be rather unreactive, and the requirements for elemental chalcogen activation usually are either a coordinatively unsaturated, strongly-reducing metal complex or a compound with a metal–metal bond.32 Nonetheless, Hayton et al. were able to perform the remarkable twoelec ...
... chalcogens, are considered to be rather unreactive, and the requirements for elemental chalcogen activation usually are either a coordinatively unsaturated, strongly-reducing metal complex or a compound with a metal–metal bond.32 Nonetheless, Hayton et al. were able to perform the remarkable twoelec ...
2012 C13 Exam answers
... dissolving 0.010 moles of HCOOH in water to make 1.0 L of solution at 298 K. Which of the following actions, considered independently, causes an increase in both the pH of the solution and the percentage ionization of HCOOH? (i) diluting with water to a final volume of 2.0 L (ii) adding 0.004 moles ...
... dissolving 0.010 moles of HCOOH in water to make 1.0 L of solution at 298 K. Which of the following actions, considered independently, causes an increase in both the pH of the solution and the percentage ionization of HCOOH? (i) diluting with water to a final volume of 2.0 L (ii) adding 0.004 moles ...