Stitching proteins into membranes, not sew simple
... first TM domain in this way may be a more widespread phenomenon than one might intuitively expect as it has also been observed to occur for short polar N-domains (24 or fewer amino acid residues) preceding a stretch of 16 leucine residues (Kocik et al., 2012). In the assembly of a potassium channel ...
... first TM domain in this way may be a more widespread phenomenon than one might intuitively expect as it has also been observed to occur for short polar N-domains (24 or fewer amino acid residues) preceding a stretch of 16 leucine residues (Kocik et al., 2012). In the assembly of a potassium channel ...
Amino acids
... is activated by E1 in an ATP-dependent fashion. E2 and E3 then work together to recognize the substrate protein and conjugate Ub to it. Ub can be attached as a monomer or as a ...
... is activated by E1 in an ATP-dependent fashion. E2 and E3 then work together to recognize the substrate protein and conjugate Ub to it. Ub can be attached as a monomer or as a ...
NMR IN DRUG DISCOVERY. FROM SCREENING TO STRUCTURE-BASED DESIGN OF
... Although protein-protein binding surfaces seem at first sight somewhat unexceptional, being relatively large (some authors estimate around 600-1200 Å2) and flat, the truth is that most display a large degree of surface and charge complementarity with their interacting partners. Moreover, contrary to ...
... Although protein-protein binding surfaces seem at first sight somewhat unexceptional, being relatively large (some authors estimate around 600-1200 Å2) and flat, the truth is that most display a large degree of surface and charge complementarity with their interacting partners. Moreover, contrary to ...
Nutrition for Endurance Athletes
... supply of carbohydrates to the body is crucial. PROTEIN Proteins are made up from as many as 20 different amino acids, whereby there are 8 essential amino acids that the body cannot synthesize. To ensure a rapid supply, amino acids and peptides (links comprising 2 – 3 amino acids) can be directly in ...
... supply of carbohydrates to the body is crucial. PROTEIN Proteins are made up from as many as 20 different amino acids, whereby there are 8 essential amino acids that the body cannot synthesize. To ensure a rapid supply, amino acids and peptides (links comprising 2 – 3 amino acids) can be directly in ...
7.3 Translation (HL ONLY)
... Translation occurs in a 5’→ 3’ direction. During translation, the ribosome moves along the mRNA towards the 3’ end. The start codon is nearer to the 5’ end. ...
... Translation occurs in a 5’→ 3’ direction. During translation, the ribosome moves along the mRNA towards the 3’ end. The start codon is nearer to the 5’ end. ...
... Most enzymes are pH dependent for their activity. Usually they have a pH-optimum which is suited to the environment in which they are generally found. Reasons for this may be that the amino acids in the active site need to be in a certain state of ionization to be active, that the substrate has to ...
Proteomic Analysis of Neisseria gonorrhoeae Biofilms
... critical to better understand the biochemical signals that regulate biofilm formation and maintenance. During the past decade, there have been various genomic [14,15] and proteomic [16] studies of biofilm formation, driven in part by technical advances in microarrays and mass spectrometry-based prot ...
... critical to better understand the biochemical signals that regulate biofilm formation and maintenance. During the past decade, there have been various genomic [14,15] and proteomic [16] studies of biofilm formation, driven in part by technical advances in microarrays and mass spectrometry-based prot ...
Phytopathogen type III effector weaponry and their plant targets
... Plant innate immunity can be divided into two branches on the basis of the types of microbial molecules recognized. One branch recognizes conserved molecules essential to many microbes called pathogen-associated molecular patterns (PAMPs) or microbe-associated molecular patterns (MAMPs). PAMP recogn ...
... Plant innate immunity can be divided into two branches on the basis of the types of microbial molecules recognized. One branch recognizes conserved molecules essential to many microbes called pathogen-associated molecular patterns (PAMPs) or microbe-associated molecular patterns (MAMPs). PAMP recogn ...
Biology 13A Lab #13: Nutrition and Digestion
... 1. Label the wells of a spot plate1 through 12 with #1 to 4 going from left to right across the top row. 2. Place 5 drops of the albumin solution in wells 1 to 4, across the top. 3. To well #2, add 5 drops of hydrochloric acid. To wells #3 and #4 add 5 drops of hydrochloric acid. To well #5, add 5 d ...
... 1. Label the wells of a spot plate1 through 12 with #1 to 4 going from left to right across the top row. 2. Place 5 drops of the albumin solution in wells 1 to 4, across the top. 3. To well #2, add 5 drops of hydrochloric acid. To wells #3 and #4 add 5 drops of hydrochloric acid. To well #5, add 5 d ...
A Rab4-like GTPase in Dictyostelium discoideum
... post-lysosomal vacuoles reacted more weakly with antiRabD antibodies than did lysosomes. Other organelles were negative for RabD. Double-label indirect immunofluorescence microscopy revealed that RabD and the 100 kDa VH+-ATPase subunit colocalized in a fine reticular network throughout the cytoplasm ...
... post-lysosomal vacuoles reacted more weakly with antiRabD antibodies than did lysosomes. Other organelles were negative for RabD. Double-label indirect immunofluorescence microscopy revealed that RabD and the 100 kDa VH+-ATPase subunit colocalized in a fine reticular network throughout the cytoplasm ...
Vesicle formation and targeting is a multi
... LDL is transferred to lysosome (fusion of vesicles from TGN) Hydrolytic enzymes cleave LDL, releasing cholesterol to cytoplasm for continued membrane biosynthesis in smooth ER Receptor is recycled back to surface (cycles about every 10 min!) ...
... LDL is transferred to lysosome (fusion of vesicles from TGN) Hydrolytic enzymes cleave LDL, releasing cholesterol to cytoplasm for continued membrane biosynthesis in smooth ER Receptor is recycled back to surface (cycles about every 10 min!) ...
Translation
... Catalyze the matching up of amino acid with the correct tRNA and using energy of ATP (activation of amino acids) Catalyzes the covalent attachment of amino acid to the 3’- OH of tRNA. ...
... Catalyze the matching up of amino acid with the correct tRNA and using energy of ATP (activation of amino acids) Catalyzes the covalent attachment of amino acid to the 3’- OH of tRNA. ...
Metabolism II
... - The reaction is characterized by a blue-violet color upon the addition of cupper sulfate to any compound containing more than 2 peptide bonds. - Biuret reagent (dilute copper sulphate in strong alkali) reacts with all compounds that contain two or more peptide bonds. - The name of the reaction is ...
... - The reaction is characterized by a blue-violet color upon the addition of cupper sulfate to any compound containing more than 2 peptide bonds. - Biuret reagent (dilute copper sulphate in strong alkali) reacts with all compounds that contain two or more peptide bonds. - The name of the reaction is ...
Lipid–protein interactions probed by electron crystallography
... monomers on the cytoplasmic side of the protein. A single PM lipid (three in total for the trimer) inserts one of its two acyl chains into this crevice to mediate interfacial ahelical packing of the protein (Figure 1C). A phosphoryl head group from this lipid in turn forms a salt bridge with the sid ...
... monomers on the cytoplasmic side of the protein. A single PM lipid (three in total for the trimer) inserts one of its two acyl chains into this crevice to mediate interfacial ahelical packing of the protein (Figure 1C). A phosphoryl head group from this lipid in turn forms a salt bridge with the sid ...
CHAPTER 17 FROM GENE TO PROTEIN Section C: The Synthesis
... pair of complimentary nucleotides with another nucleotide pair is called a base-pair substitution. • Some base-pair substitutions have little or no impact on protein function. • In silent mutations, alterations of nucleotides still indicate the same amino acids because of redundancy in the genetic c ...
... pair of complimentary nucleotides with another nucleotide pair is called a base-pair substitution. • Some base-pair substitutions have little or no impact on protein function. • In silent mutations, alterations of nucleotides still indicate the same amino acids because of redundancy in the genetic c ...
17C-SynthesisOfProtein
... pair of complimentary nucleotides with another nucleotide pair is called a base-pair substitution. • Some base-pair substitutions have little or no impact on protein function. • In silent mutations, alterations of nucleotides still indicate the same amino acids because of redundancy in the genetic c ...
... pair of complimentary nucleotides with another nucleotide pair is called a base-pair substitution. • Some base-pair substitutions have little or no impact on protein function. • In silent mutations, alterations of nucleotides still indicate the same amino acids because of redundancy in the genetic c ...
17C-SynthesisOfProtein
... pair of complimentary nucleotides with another nucleotide pair is called a base-pair substitution. • Some base-pair substitutions have little or no impact on protein function. • In silent mutations, alterations of nucleotides still indicate the same amino acids because of redundancy in the genetic c ...
... pair of complimentary nucleotides with another nucleotide pair is called a base-pair substitution. • Some base-pair substitutions have little or no impact on protein function. • In silent mutations, alterations of nucleotides still indicate the same amino acids because of redundancy in the genetic c ...
Chapter 17 Protein Synthesis
... • During and after synthesis, a polypeptide chain spontaneously coils and folds into its threedimensional shape • Proteins may also require post-translational modifications before doing their job • Some polypeptides are activated by enzymes that cleave them • Other polypeptides come together to form ...
... • During and after synthesis, a polypeptide chain spontaneously coils and folds into its threedimensional shape • Proteins may also require post-translational modifications before doing their job • Some polypeptides are activated by enzymes that cleave them • Other polypeptides come together to form ...
Chapter 5 Chemical messengers
... (CBG) for cortisol Other carrier protein—for example, albumin—are not specific and can transport many different hormones Hormones that are present in dissolved form have relatively short half-lives 半衰期短, usually minutes Hormones that are bound to carrier proteins are protected from degradation ...
... (CBG) for cortisol Other carrier protein—for example, albumin—are not specific and can transport many different hormones Hormones that are present in dissolved form have relatively short half-lives 半衰期短, usually minutes Hormones that are bound to carrier proteins are protected from degradation ...
Chapter 5-化學訊息傳導物檔案
... (CBG) for cortisol Other carrier protein—for example, albumin—are not specific and can transport many different hormones ...
... (CBG) for cortisol Other carrier protein—for example, albumin—are not specific and can transport many different hormones ...
The rhythm of protein synthesis does not depend on oscillations of
... the activity of ornithine decarboxylase, a key enzyme of polyamine synthesis, in hepatocytes in vitro (Jarigin et a!., 1978). In these two systems a rhythm of cyclic AMP levels has also been described (Jarigin et al., 1979; Ishida and Jasumasu, 1982). Oscillations of total adenylic nucleotide conten ...
... the activity of ornithine decarboxylase, a key enzyme of polyamine synthesis, in hepatocytes in vitro (Jarigin et a!., 1978). In these two systems a rhythm of cyclic AMP levels has also been described (Jarigin et al., 1979; Ishida and Jasumasu, 1982). Oscillations of total adenylic nucleotide conten ...
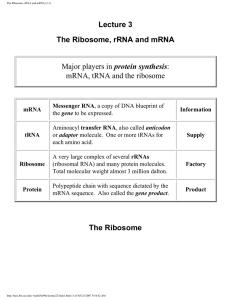
The Ribosome, rRNA and mRNA (3.1)
... Using the atomic structures of the large ribosomal subunit from Haloarcula marismortui and its complexes with two substrate analogs, we establish that the ribosome is a ribozyme and address the catalytic properties of its all-RNA active site. Both substrate analogs are contacted exclusively by conse ...
... Using the atomic structures of the large ribosomal subunit from Haloarcula marismortui and its complexes with two substrate analogs, we establish that the ribosome is a ribozyme and address the catalytic properties of its all-RNA active site. Both substrate analogs are contacted exclusively by conse ...
Co-opting sulphur-carrier proteins from primary metabolic pathways
... BE-7585A cluster. Subsequent genome sequencing uncovered a few genes encoding sulphur-carrier proteins that are probably involved in the biosynthesis of primary metabolites but only one activating enzyme gene in the A. orientalis genome. Further experiments showed that this activating enzyme can ade ...
... BE-7585A cluster. Subsequent genome sequencing uncovered a few genes encoding sulphur-carrier proteins that are probably involved in the biosynthesis of primary metabolites but only one activating enzyme gene in the A. orientalis genome. Further experiments showed that this activating enzyme can ade ...
Protein

Proteins (/ˈproʊˌtiːnz/ or /ˈproʊti.ɨnz/) are large biomolecules, or macromolecules, consisting of one or more long chains of amino acid residues. Proteins perform a vast array of functions within living organisms, including catalyzing metabolic reactions, DNA replication, responding to stimuli, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific three-dimensional structure that determines its activity.A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than about 20-30 residues, are rarely considered to be proteins and are commonly called peptides, or sometimes oligopeptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residues in a protein is defined by the sequence of a gene, which is encoded in the genetic code. In general, the genetic code specifies 20 standard amino acids; however, in certain organisms the genetic code can include selenocysteine and—in certain archaea—pyrrolysine. Shortly after or even during synthesis, the residues in a protein are often chemically modified by posttranslational modification, which alters the physical and chemical properties, folding, stability, activity, and ultimately, the function of the proteins. Sometimes proteins have non-peptide groups attached, which can be called prosthetic groups or cofactors. Proteins can also work together to achieve a particular function, and they often associate to form stable protein complexes.Once formed, proteins only exist for a certain period of time and are then degraded and recycled by the cell's machinery through the process of protein turnover. A protein's lifespan is measured in terms of its half-life and covers a wide range. They can exist for minutes or years with an average lifespan of 1–2 days in mammalian cells. Abnormal and or misfolded proteins are degraded more rapidly either due to being targeted for destruction or due to being unstable.Like other biological macromolecules such as polysaccharides and nucleic acids, proteins are essential parts of organisms and participate in virtually every process within cells. Many proteins are enzymes that catalyze biochemical reactions and are vital to metabolism. Proteins also have structural or mechanical functions, such as actin and myosin in muscle and the proteins in the cytoskeleton, which form a system of scaffolding that maintains cell shape. Other proteins are important in cell signaling, immune responses, cell adhesion, and the cell cycle. Proteins are also necessary in animals' diets, since animals cannot synthesize all the amino acids they need and must obtain essential amino acids from food. Through the process of digestion, animals break down ingested protein into free amino acids that are then used in metabolism.Proteins may be purified from other cellular components using a variety of techniques such as ultracentrifugation, precipitation, electrophoresis, and chromatography; the advent of genetic engineering has made possible a number of methods to facilitate purification. Methods commonly used to study protein structure and function include immunohistochemistry, site-directed mutagenesis, X-ray crystallography, nuclear magnetic resonance and mass spectrometry.