chemistry-study-guide-grade
... 1. Provide the meaning of each type of quantum number (principal, angular momentum, magnetic and electron spin). 2. Apply quantum number rules to determine allowable values for each type of quantum number. 3. Understand the basis of atomic orbitals. 4. Arrange atomic orbitals based upon energy level ...
... 1. Provide the meaning of each type of quantum number (principal, angular momentum, magnetic and electron spin). 2. Apply quantum number rules to determine allowable values for each type of quantum number. 3. Understand the basis of atomic orbitals. 4. Arrange atomic orbitals based upon energy level ...
Unit 2 Intro Worksheet - Coral Gables Senior High
... 3. What is the quantum mechanical model? 4. Explain what is meant by the Heisenberg uncertainty principle. 5. Explain the three principles that govern the electron configuration in an atom. Matching Match each item with the correct statement below. a. atomic orbital ...
... 3. What is the quantum mechanical model? 4. Explain what is meant by the Heisenberg uncertainty principle. 5. Explain the three principles that govern the electron configuration in an atom. Matching Match each item with the correct statement below. a. atomic orbital ...
7.4 The Wave Nature of Matter * 7.5 Quantum Mechanics and the Atom
... • Since we can not determine the exact location and velocity of an electron at the same time, experimentation has been done over time to identify the most likely places that the electrons exist in an atom. These locations are called orbitals. • Schrödinger's equation can be used derive the energies ...
... • Since we can not determine the exact location and velocity of an electron at the same time, experimentation has been done over time to identify the most likely places that the electrons exist in an atom. These locations are called orbitals. • Schrödinger's equation can be used derive the energies ...
Chapter 2 Learning Objectives
... a. The threshold frequency b. The correlation between radiant intensity and the number of emitted electrons 3. Understand that the electrons of an atom behave as waves, resulting in quantum numbers 4. Know all four quantum numbers (n, l, ml, ms), and the dependency rules between them 5. Be able to u ...
... a. The threshold frequency b. The correlation between radiant intensity and the number of emitted electrons 3. Understand that the electrons of an atom behave as waves, resulting in quantum numbers 4. Know all four quantum numbers (n, l, ml, ms), and the dependency rules between them 5. Be able to u ...
Answers to Critical Thinking Questions 4
... were added to 4s (violating the Aufbau principle). The correct configuration is 1s22s22p63s23p5 b) 1s22s22p63s23p7 – the maximum number of electrons in 3p is 6 (violating the Pauli exclusion principle). The correct configuration is 1s22s22p63s23p64s1 c) 1s22s22p63s23p63d2 – the 4s orbital has a lowe ...
... were added to 4s (violating the Aufbau principle). The correct configuration is 1s22s22p63s23p5 b) 1s22s22p63s23p7 – the maximum number of electrons in 3p is 6 (violating the Pauli exclusion principle). The correct configuration is 1s22s22p63s23p64s1 c) 1s22s22p63s23p63d2 – the 4s orbital has a lowe ...
WAVE MECHANICS (Schrödinger, 1926)
... of matter and the uncertainty principle. * The state of an electron is described by a function y, called the “wave function”. * y can be obtained by solving Schrödinger’s equation (a differential equation): Hy=Ey ...
... of matter and the uncertainty principle. * The state of an electron is described by a function y, called the “wave function”. * y can be obtained by solving Schrödinger’s equation (a differential equation): Hy=Ey ...
Chapter Outline Measuring Polarity Examples Permanent Dipole
... around the central C atom is tetrahedral. ...
... around the central C atom is tetrahedral. ...
Chapt7
... Energetically excited atoms only emit radiation in discrete energies corresponding to the atom's electronic energy levels. (see Figure 7.11) ...
... Energetically excited atoms only emit radiation in discrete energies corresponding to the atom's electronic energy levels. (see Figure 7.11) ...
Section 5-2
... Continued • Electrons move in certain, specific, circular orbitals • Smaller orbit = lower energy level • Assigned the allowable electron orbitals the principle quantum number, n. • 1st orbit= lowest energy: n=1 • 2nd orbit= 2nd lowest energy: n=2 ...
... Continued • Electrons move in certain, specific, circular orbitals • Smaller orbit = lower energy level • Assigned the allowable electron orbitals the principle quantum number, n. • 1st orbit= lowest energy: n=1 • 2nd orbit= 2nd lowest energy: n=2 ...
How are quantum numbers used to describe electrons
... How many orbitals in the 4th energy level? How many electrons can be in the 4th energy level? For the known elements, ________ orbitals and ______ electrons is the maximum number in energy levels 5-7. What rules are used to explain how electrons fill orbitals? Pauli exclusion principle—no two electr ...
... How many orbitals in the 4th energy level? How many electrons can be in the 4th energy level? For the known elements, ________ orbitals and ______ electrons is the maximum number in energy levels 5-7. What rules are used to explain how electrons fill orbitals? Pauli exclusion principle—no two electr ...
apch07_quantum
... f) Label each of the orbital pictures found in question 78 (page 329)with the appropriate letter: g) When n=5, the possible values of l are ______. h) The maximum number of orbitals that can be assigned to the n=4 shell is ____. ...
... f) Label each of the orbital pictures found in question 78 (page 329)with the appropriate letter: g) When n=5, the possible values of l are ______. h) The maximum number of orbitals that can be assigned to the n=4 shell is ____. ...
South Pasadena · Chemistry
... f) Label each of the orbital pictures found in question 78 (page 329)with the appropriate letter: g) When n=5, the possible values of l are ______. h) The maximum number of orbitals that can be assigned to the n=4 shell is ____. ...
... f) Label each of the orbital pictures found in question 78 (page 329)with the appropriate letter: g) When n=5, the possible values of l are ______. h) The maximum number of orbitals that can be assigned to the n=4 shell is ____. ...
Document
... Chemistry 130 (Lecture VII-VIII) Answer 1. Which of the following statements is not consistent with a quantum mechanical view of nature? a. Matter can be thought of as waves b. Excited atoms can emit all possible energies c. Knowing the exact speed of an electron means we do not know anything about ...
... Chemistry 130 (Lecture VII-VIII) Answer 1. Which of the following statements is not consistent with a quantum mechanical view of nature? a. Matter can be thought of as waves b. Excited atoms can emit all possible energies c. Knowing the exact speed of an electron means we do not know anything about ...
Hydroperoxide ion P.9 is much less basic than hydroxide ion P.10
... If all molecular orbitals were filled, then there would have to be one electron in each spin state on each atom, and the sum of the squares of all the c values on any one atom in all the molecular orbitals must also equal one. Thus the σ*-antibonding orbital of hydrogen will have c-values of 0.707 a ...
... If all molecular orbitals were filled, then there would have to be one electron in each spin state on each atom, and the sum of the squares of all the c values on any one atom in all the molecular orbitals must also equal one. Thus the σ*-antibonding orbital of hydrogen will have c-values of 0.707 a ...
Quantum Mechanical Model
... Quantum Mechanical Model • As the energy of an electron increases, so does the quantum number (n) • Each principle energy level is also split up into one or more sublevels • Chart on Pg. 145 [http://www.chemistry.mcmaster.ca/esam/Chapter_4/fig4-2.jpg] ...
... Quantum Mechanical Model • As the energy of an electron increases, so does the quantum number (n) • Each principle energy level is also split up into one or more sublevels • Chart on Pg. 145 [http://www.chemistry.mcmaster.ca/esam/Chapter_4/fig4-2.jpg] ...
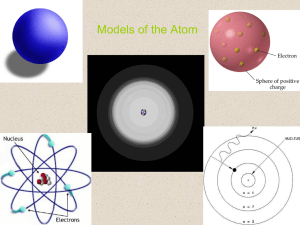
Models of the Atom
... • The modern description of electrons in an atom • Determines the allowed energies an electron can have and how likely it is to find the electron in various locations around the nucleus. • Based on probability ...
... • The modern description of electrons in an atom • Determines the allowed energies an electron can have and how likely it is to find the electron in various locations around the nucleus. • Based on probability ...
South Pasadena · Chemistry
... The principal quantum number, n, can have the values of: ___ ___ ___ ___ ___, etc. The angular momentum quantum number, l, can have integer values from ______ to ______. The magnetic quantum number, ml, can have integer values from _____ to _____. 2. When n = 3, l can have values of ________________ ...
... The principal quantum number, n, can have the values of: ___ ___ ___ ___ ___, etc. The angular momentum quantum number, l, can have integer values from ______ to ______. The magnetic quantum number, ml, can have integer values from _____ to _____. 2. When n = 3, l can have values of ________________ ...
Molecular orbital

In chemistry, a molecular orbital (or MO) is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term orbital was introduced by Robert S. Mulliken in 1932 as an abbreviation for one-electron orbital wave function. At an elementary level, it is used to describe the region of space in which the function has a significant amplitude. Molecular orbitals are usually constructed by combining atomic orbitals or hybrid orbitals from each atom of the molecule, or other molecular orbitals from groups of atoms. They can be quantitatively calculated using the Hartree–Fock or self-consistent field (SCF) methods.