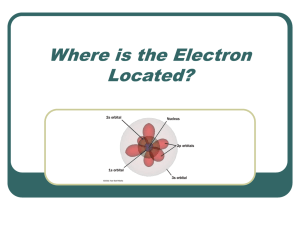
Quantum Mechanical Model - Elmwood Park Memorial Middle School
... • Take out your answers to the introduc4on reading. • These slides are on the webpage if you would like them. ...
... • Take out your answers to the introduc4on reading. • These slides are on the webpage if you would like them. ...
Molecular Geometry and Chemical Bonding Theory
... The dipole moment is a measure of the degree of charge separation in a molecule We can view the polarity of individual bonds with in a molecule as vector quantities. Measurements of dipole moments are based on the fact that polar molecules can be oriented by an electric field. Thus molecules that ar ...
... The dipole moment is a measure of the degree of charge separation in a molecule We can view the polarity of individual bonds with in a molecule as vector quantities. Measurements of dipole moments are based on the fact that polar molecules can be oriented by an electric field. Thus molecules that ar ...
Details
... General Chemistry (CHM 111): MD (CHM 111), Phar (PH xxx) , (Dent Den xxx) Proposed by Dr. Badereldeen (Revised 2012) Week ...
... General Chemistry (CHM 111): MD (CHM 111), Phar (PH xxx) , (Dent Den xxx) Proposed by Dr. Badereldeen (Revised 2012) Week ...
Chapter 3
... Solving the wave equation gives a set of wave functions, or orbitals and their corresponding energies. Each orbitals describes a spatial distribution of electron density. An orbitals is described by a set of three quantum numbers. ...
... Solving the wave equation gives a set of wave functions, or orbitals and their corresponding energies. Each orbitals describes a spatial distribution of electron density. An orbitals is described by a set of three quantum numbers. ...
Review Sheet
... Endothermic vs. exothermic Stoichiometry using energy (using enthalpy of reaction and balanced chemical reactions) Calculating H using Hess’s Law, Enthalpy Diagrams, and/or H° of formations Calorimetry calculations to determine H and q Standard States and enthalpy of formation Using Hess’s Law eq ...
... Endothermic vs. exothermic Stoichiometry using energy (using enthalpy of reaction and balanced chemical reactions) Calculating H using Hess’s Law, Enthalpy Diagrams, and/or H° of formations Calorimetry calculations to determine H and q Standard States and enthalpy of formation Using Hess’s Law eq ...
Chapter7_1 - Department of Chemistry [FSU]
... De Broglie: electrons are waves matter is wavelike: ...
... De Broglie: electrons are waves matter is wavelike: ...
3: Many electrons
... Ψtot(r1, r2 , · · · rN) = (φa (r1)φb (r2) · · · φn (rN))space × [Φ(1)Φ(2) · · · Φ(N)]spin . Here, the φi are the molecular orbitals (e.g., the 1σg orbital of H+ 2 just discussed). The total wavefunction is associated with a particular electron configuration. ...
... Ψtot(r1, r2 , · · · rN) = (φa (r1)φb (r2) · · · φn (rN))space × [Φ(1)Φ(2) · · · Φ(N)]spin . Here, the φi are the molecular orbitals (e.g., the 1σg orbital of H+ 2 just discussed). The total wavefunction is associated with a particular electron configuration. ...
Molecular Geometry and Chemical Bonding Theory
... The total number of electrons can't be more than two. Strength of the bond depends on the orbital overlap Hybrid orbitals - bonding that are obtained by taking combinations of atomic orbitals of the isolated atoms. The number of hybrid orbitals formed always equals the number of atomic orbitals used ...
... The total number of electrons can't be more than two. Strength of the bond depends on the orbital overlap Hybrid orbitals - bonding that are obtained by taking combinations of atomic orbitals of the isolated atoms. The number of hybrid orbitals formed always equals the number of atomic orbitals used ...
HOMEWORK 4-4 - losbanosusd.org
... STANDARDIZED TEST PREP Circle the letter of the best answer. 1. Which of the following indicates the s sublevel in the third main energy level? a. s3 b. 3xyz ...
... STANDARDIZED TEST PREP Circle the letter of the best answer. 1. Which of the following indicates the s sublevel in the third main energy level? a. s3 b. 3xyz ...
BCIT Fall 2012 Chem 3615 Exam #2
... If a1 and a2 are constants, x) and 2(x) are functions, and  is a Hermitian operator that satisfy the equation, Âx) = a1(x) and Â2(x) = a22(x) where a1 a2. Which of the following statements is false? (x) is an eigenfunction of  . If (x) = (x) + (x) then (x) is an eigenfunc ...
... If a1 and a2 are constants, x) and 2(x) are functions, and  is a Hermitian operator that satisfy the equation, Âx) = a1(x) and Â2(x) = a22(x) where a1 a2. Which of the following statements is false? (x) is an eigenfunction of  . If (x) = (x) + (x) then (x) is an eigenfunc ...
Text Questions - Teach-n-Learn-Chem
... exists between two atoms a _________ domain. When two electrons are located principally on one atom, they constitute a ______________ (or _______) ______. 7. The three things that produce an electron domain around a central atom are… ...
... exists between two atoms a _________ domain. When two electrons are located principally on one atom, they constitute a ______________ (or _______) ______. 7. The three things that produce an electron domain around a central atom are… ...
Chapter 7, Quantum Nos.
... value penetrate closer to the nucleus and are less shielded and have a lower energy than those with a higher l value. The result is that for a given value of n the energy order is s < p < d < f. Orbitals are filled from lowest energy to highest energy. Each orbital holds 2 electrons (Pauli Exclusion ...
... value penetrate closer to the nucleus and are less shielded and have a lower energy than those with a higher l value. The result is that for a given value of n the energy order is s < p < d < f. Orbitals are filled from lowest energy to highest energy. Each orbital holds 2 electrons (Pauli Exclusion ...
Chp7,Quantum_Num
... value penetrate closer to the nucleus and are less shielded and have a lower energy than those with a higher l value. The result is that for a given value of n the energy order is s < p < d < f. Orbitals are filled from lowest energy to highest energy. Each orbital holds 2 electrons (Pauli Exclusion ...
... value penetrate closer to the nucleus and are less shielded and have a lower energy than those with a higher l value. The result is that for a given value of n the energy order is s < p < d < f. Orbitals are filled from lowest energy to highest energy. Each orbital holds 2 electrons (Pauli Exclusion ...
CM1111* Question 1 (40 marks) Multiple Choice Questions, 5 marks
... D. For an s orbital, at a particular distance from the nucleus, the probability of finding the electron is equal in all directions. (5) Which of the following statement(s) is/are not true? A. The node of an orbital refers to the region where its wavefunction is zero. B. To find the energy of the ele ...
... D. For an s orbital, at a particular distance from the nucleus, the probability of finding the electron is equal in all directions. (5) Which of the following statement(s) is/are not true? A. The node of an orbital refers to the region where its wavefunction is zero. B. To find the energy of the ele ...
Electron Configuration
... 3 methods: 1. Energy-Level Diagrams (orbital diagram) 2. Complete electron configuration 3. Condensed electron configuration (AKA noble gas configuration) ...
... 3 methods: 1. Energy-Level Diagrams (orbital diagram) 2. Complete electron configuration 3. Condensed electron configuration (AKA noble gas configuration) ...
Molecular orbital

In chemistry, a molecular orbital (or MO) is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term orbital was introduced by Robert S. Mulliken in 1932 as an abbreviation for one-electron orbital wave function. At an elementary level, it is used to describe the region of space in which the function has a significant amplitude. Molecular orbitals are usually constructed by combining atomic orbitals or hybrid orbitals from each atom of the molecule, or other molecular orbitals from groups of atoms. They can be quantitatively calculated using the Hartree–Fock or self-consistent field (SCF) methods.









![Chapter7_1 - Department of Chemistry [FSU]](http://s1.studyres.com/store/data/016128835_1-aea3c1aec04363d6cbf538e8faf80e45-300x300.png)