Superconcepts
... 1. An atom’s bonding & reactive properties are determined by electron configuration. 2. The behavior of electrons in atoms is dictated by quantum mechanics. Concepts a. Newtonian physics don’t accurately describe the behavior of matter at the subatomic level. b. Both light and electrons behave as wa ...
... 1. An atom’s bonding & reactive properties are determined by electron configuration. 2. The behavior of electrons in atoms is dictated by quantum mechanics. Concepts a. Newtonian physics don’t accurately describe the behavior of matter at the subatomic level. b. Both light and electrons behave as wa ...
e- are outside nucleus nucleus
... - quantized energy levels) • Difference: e- do not travel in fixed paths; they exist in an e- cloud e- cloud: region around the nucleus where the probability of finding an e- is about 90% ...
... - quantized energy levels) • Difference: e- do not travel in fixed paths; they exist in an e- cloud e- cloud: region around the nucleus where the probability of finding an e- is about 90% ...
Chem1101 – Semester 1
... Heteronuclear molecules: are formed through the mixing of different atomic orbitals gives rise to asymmetric molecular orbitals The bond between two elements with different electronegativities will be polar. Th ...
... Heteronuclear molecules: are formed through the mixing of different atomic orbitals gives rise to asymmetric molecular orbitals The bond between two elements with different electronegativities will be polar. Th ...
eprint_11_28683_250
... position of the electron at the same instant in time. This is a statement of Heisenberg’s uncertainty principle. In order to get around this problem, rather than trying to define its exact position and momentum, we use the probability of finding the electron in a given volume of space. The probabil ...
... position of the electron at the same instant in time. This is a statement of Heisenberg’s uncertainty principle. In order to get around this problem, rather than trying to define its exact position and momentum, we use the probability of finding the electron in a given volume of space. The probabil ...
AP Chapter 9 Molecular Shapes
... π bonds • π bonds must lie in the same plane, therefore, the presence of π bonds makes the molecule slightly rigid. ...
... π bonds • π bonds must lie in the same plane, therefore, the presence of π bonds makes the molecule slightly rigid. ...
VSEPR Power Point
... • Valence Bond Theory: a quantum mechanical description of bonding that pictures covalent bond formation as the overlap of two singly occupied atomic orbitals. • VSEPR effective but ignores the orbital concepts discussed in quantum mechanics. • H2 forms due to overlap of two 1s orbitals. • Electron ...
... • Valence Bond Theory: a quantum mechanical description of bonding that pictures covalent bond formation as the overlap of two singly occupied atomic orbitals. • VSEPR effective but ignores the orbital concepts discussed in quantum mechanics. • H2 forms due to overlap of two 1s orbitals. • Electron ...
Matter and Energy Identify a chemical physical change Identify a
... Heisenberg Uncertainty Principle Hund’s rule Pauli exclusion Principle Ground and excited state Sublevels s p d f ...
... Heisenberg Uncertainty Principle Hund’s rule Pauli exclusion Principle Ground and excited state Sublevels s p d f ...
Electrons in the Atom
... This similar configuration causes them to behave the same chemically. It’s for that reason they are in the same family or group on the periodic table. Each group will have the same ending configuration, in this case something that ends in s1. ...
... This similar configuration causes them to behave the same chemically. It’s for that reason they are in the same family or group on the periodic table. Each group will have the same ending configuration, in this case something that ends in s1. ...
Chemical Building Blocks
... QUIZ: ½ sheet of paper An analyst measured a 3.3 x 10-2 L sample and found it weighed 385 mg. Report its density in g/mL. (Proper SF) Give an example of the ff. ...
... QUIZ: ½ sheet of paper An analyst measured a 3.3 x 10-2 L sample and found it weighed 385 mg. Report its density in g/mL. (Proper SF) Give an example of the ff. ...
Writing Electron Configuration
... It’s useful to be able to write out the location of electrons in an atom. Si: 1s2, 2s2, 2p6, 3s2, 3p2 ...
... It’s useful to be able to write out the location of electrons in an atom. Si: 1s2, 2s2, 2p6, 3s2, 3p2 ...
Atomic structure ls on a periodic table.
... Atomic structure ls on a periodic table. -metals, and noble gases are found on a periodic table. Specify how to predict the charge expected for the most stable ion of an atom. the line spectra of atomic species relate to the idea of quantized states of electrons in atoms. A Jablonksi diagram may be ...
... Atomic structure ls on a periodic table. -metals, and noble gases are found on a periodic table. Specify how to predict the charge expected for the most stable ion of an atom. the line spectra of atomic species relate to the idea of quantized states of electrons in atoms. A Jablonksi diagram may be ...
4.6 Quantum Mechanics and Bonding Hybridization
... • Valence bond theory is a theory stating that atomic orbitals overlap to form a new orbital with a pair of opposite spin electrons – A covalent bond forms when 2 atomic orbitals, each with an unpaired electron, overlap – When the covalent bond forms, the lowest energy state is obtained when partici ...
... • Valence bond theory is a theory stating that atomic orbitals overlap to form a new orbital with a pair of opposite spin electrons – A covalent bond forms when 2 atomic orbitals, each with an unpaired electron, overlap – When the covalent bond forms, the lowest energy state is obtained when partici ...
Unit 1 Inorganic Flashcards
... ion is attached to a group of surrounding molecules or ions by dative covalent bonds (also known as coordinate bonds). ...
... ion is attached to a group of surrounding molecules or ions by dative covalent bonds (also known as coordinate bonds). ...
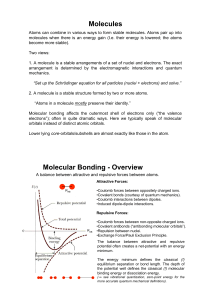
Molecules Molecular Bonding
... 1. A molecule is a stable arrangements of a set of nuclei and electrons. The exact arrangement is determined by the electromagnetic interactions and quantum mechanics. “Set up the Schrödinger equation for all particles (nuclei + electrons) and solve.” 2. A molecule is a stable structure formed by tw ...
... 1. A molecule is a stable arrangements of a set of nuclei and electrons. The exact arrangement is determined by the electromagnetic interactions and quantum mechanics. “Set up the Schrödinger equation for all particles (nuclei + electrons) and solve.” 2. A molecule is a stable structure formed by tw ...
Molecular orbital

In chemistry, a molecular orbital (or MO) is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term orbital was introduced by Robert S. Mulliken in 1932 as an abbreviation for one-electron orbital wave function. At an elementary level, it is used to describe the region of space in which the function has a significant amplitude. Molecular orbitals are usually constructed by combining atomic orbitals or hybrid orbitals from each atom of the molecule, or other molecular orbitals from groups of atoms. They can be quantitatively calculated using the Hartree–Fock or self-consistent field (SCF) methods.