Modern Model of the Atom Student Notes and Assignment
... 1. AUFBAU PRINCIPLE - electrons enter orbitals of the lowest energy levels first 2. PAULI EXCLUSION PRINCIPLE - an atomic orbital may hold at most two electrons. Each must have an opposite spin. 3. HUND’S RULE - when electrons occupy orbitals of equal energy one electron enters each orbital until al ...
... 1. AUFBAU PRINCIPLE - electrons enter orbitals of the lowest energy levels first 2. PAULI EXCLUSION PRINCIPLE - an atomic orbital may hold at most two electrons. Each must have an opposite spin. 3. HUND’S RULE - when electrons occupy orbitals of equal energy one electron enters each orbital until al ...
2·QUIZLET VOCABULARY: Quantum Numbers Study online at
... 2. Aufbau principal: states that each electron occupies the lowest energy orbital available 3. electron configuration: the arrangement of electrons around nucleus in an atom 4. Hunds rule: orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron, ...
... 2. Aufbau principal: states that each electron occupies the lowest energy orbital available 3. electron configuration: the arrangement of electrons around nucleus in an atom 4. Hunds rule: orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron, ...
Chemistry 215 Quiz 1 (20 points)
... According to MO theory, overlap of two s atomic orbitals produces a) one bonding molecular orbital and one hybrid orbital b) two bonding molecular orbitals c) two bonding molecular orbitals and two antibonding molecular orbitals d) two bonding molecular orbitals and one antibonding molecular orbital ...
... According to MO theory, overlap of two s atomic orbitals produces a) one bonding molecular orbital and one hybrid orbital b) two bonding molecular orbitals c) two bonding molecular orbitals and two antibonding molecular orbitals d) two bonding molecular orbitals and one antibonding molecular orbital ...
5. Quantum mechanics of chemical binding
... u1 is a binding orbital, u2 is anti-bonding (meaning it eases the bond). Prinzipal question: what is chemical bond according to quantum mechanics? Answer from the above figures: • according to the energy curves: energy decreases if the two atoms get closer; • form of the orbitals and the density: th ...
... u1 is a binding orbital, u2 is anti-bonding (meaning it eases the bond). Prinzipal question: what is chemical bond according to quantum mechanics? Answer from the above figures: • according to the energy curves: energy decreases if the two atoms get closer; • form of the orbitals and the density: th ...
Answers to Critical Thinking Questions 4
... The 2s has one radial node and the 3s has two radial nodes. 3p have one radial node. In general, the number of radial nodes is equal to n – l - 1. ...
... The 2s has one radial node and the 3s has two radial nodes. 3p have one radial node. In general, the number of radial nodes is equal to n – l - 1. ...
OBJECTIVE WORKSHEET Quantum Theory 1. How did
... 3. How many energy levels for electrons does the chapter discuss? 4. Who discovered the QUANTUM MECHANICAL MODEL? 5. What shape does the s and p orbitals have? 6. What does "n" stand for when we discuss atomic orbitals? 7. What is the maximum number of electrons allowed in when n=4? 8. What is "neon ...
... 3. How many energy levels for electrons does the chapter discuss? 4. Who discovered the QUANTUM MECHANICAL MODEL? 5. What shape does the s and p orbitals have? 6. What does "n" stand for when we discuss atomic orbitals? 7. What is the maximum number of electrons allowed in when n=4? 8. What is "neon ...
Quantum Mechanical Model of the Atom
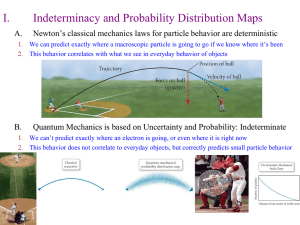
... MODEL OF THE ATOM ESSENTIAL QUESTION: WHAT IS THE CURRENT MODEL OF THE ATOM? ...
... MODEL OF THE ATOM ESSENTIAL QUESTION: WHAT IS THE CURRENT MODEL OF THE ATOM? ...
الرقم الجامعي
... Q1- Which of the following electrons has the highest effective nuclear charge (Z*): a 3d and 4s electron in Mn or Co? -------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- ...
... Q1- Which of the following electrons has the highest effective nuclear charge (Z*): a 3d and 4s electron in Mn or Co? -------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- ...
Atomic Orbitals - Daytona State College
... Atomic Orbitals The electron orbitals presented here represent a volume of space within which an electron would have a certain probability. For example, in a simple lowest-energy state hydrogen atom, the electrons are most likely to be found within a sphere around the nucleus of an atom. In a higher ...
... Atomic Orbitals The electron orbitals presented here represent a volume of space within which an electron would have a certain probability. For example, in a simple lowest-energy state hydrogen atom, the electrons are most likely to be found within a sphere around the nucleus of an atom. In a higher ...
Chapter 7 Handout 1 Atomic Orbitals Quantum Numbers: Principal
... Rules for filling orbitals: 1. Aufbau Principle: a. Electrons fill up orbitals of lowest energy first b. Orbitals in the same sublevel are equal in energy c. Sometimes energy levels overlap 2. Pauli Exculsion Principle a. There is a max of 2 electrons in any one orbital b. These 2 electrons must ha ...
... Rules for filling orbitals: 1. Aufbau Principle: a. Electrons fill up orbitals of lowest energy first b. Orbitals in the same sublevel are equal in energy c. Sometimes energy levels overlap 2. Pauli Exculsion Principle a. There is a max of 2 electrons in any one orbital b. These 2 electrons must ha ...
Quantum numbers
... – is mathematically imprecise but chemically highly successful, and graphically simple because intuitive – Syntax: • radially symmetric molecular orbitals are named (sigma) orbitals • rotationally symmetric molecular orbitals are named (pi) orbitals • a “b” stands for bonding, a “*” for anti-bonding ...
... – is mathematically imprecise but chemically highly successful, and graphically simple because intuitive – Syntax: • radially symmetric molecular orbitals are named (sigma) orbitals • rotationally symmetric molecular orbitals are named (pi) orbitals • a “b” stands for bonding, a “*” for anti-bonding ...
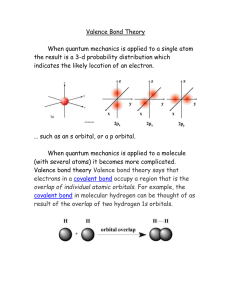
Valence Bond Theory
... When quantum mechanics is applied to a molecule (with several atoms) it becomes more complicated. Valence bond theory Valence bond theory says that electrons in a covalent bond occupy a region that is the overlap of individual atomic orbitals. For example, the covalent bond in molecular hydrogen can ...
... When quantum mechanics is applied to a molecule (with several atoms) it becomes more complicated. Valence bond theory Valence bond theory says that electrons in a covalent bond occupy a region that is the overlap of individual atomic orbitals. For example, the covalent bond in molecular hydrogen can ...
Molecular orbital

In chemistry, a molecular orbital (or MO) is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term orbital was introduced by Robert S. Mulliken in 1932 as an abbreviation for one-electron orbital wave function. At an elementary level, it is used to describe the region of space in which the function has a significant amplitude. Molecular orbitals are usually constructed by combining atomic orbitals or hybrid orbitals from each atom of the molecule, or other molecular orbitals from groups of atoms. They can be quantitatively calculated using the Hartree–Fock or self-consistent field (SCF) methods.