time-dependent density functional theoretical - Prof. Shih
... on the earlier fundamental work of Hohenberg and Kohn [6] and Kohn and Sham [7]. In the Kohn-Sham DFT formalism [7], the electron density is decomposed into a set of orbitals, leading to a set of one-electron Schrödinger-like equations to be solved self-consistently. The Kohn-Sham equations are str ...
... on the earlier fundamental work of Hohenberg and Kohn [6] and Kohn and Sham [7]. In the Kohn-Sham DFT formalism [7], the electron density is decomposed into a set of orbitals, leading to a set of one-electron Schrödinger-like equations to be solved self-consistently. The Kohn-Sham equations are str ...
Document
... Allowed values for K and J: both must, by conditions of quantum mechanics, be integral or zero. The total angular momentum can be as large as we like – i.e., (except that a real molecule will be disrupted at very high rotational speeds) Once we have chosen J, however, K is more limited. ...
... Allowed values for K and J: both must, by conditions of quantum mechanics, be integral or zero. The total angular momentum can be as large as we like – i.e., (except that a real molecule will be disrupted at very high rotational speeds) Once we have chosen J, however, K is more limited. ...
The quantum atom
... standing waves in mechanical systems did not really answer the question; the electron is still a particle having a negative charge and is attracted to the nucleus. The answer comes from the Heisenberg uncertainty principle, which says that a quantum particle such as the electron cannot simultaneousl ...
... standing waves in mechanical systems did not really answer the question; the electron is still a particle having a negative charge and is attracted to the nucleus. The answer comes from the Heisenberg uncertainty principle, which says that a quantum particle such as the electron cannot simultaneousl ...
3. chemical bonding and molecular structure
... • This theory explains how and why the bonds are formed. • Valence electrons are responsible for bonding process. • Inert gases have ns2 np6 configuration but, Helium has 1s2. Thus, all inert gases have octet and helium has duplet configuration. • Noble gases are chemically inert and will not take p ...
... • This theory explains how and why the bonds are formed. • Valence electrons are responsible for bonding process. • Inert gases have ns2 np6 configuration but, Helium has 1s2. Thus, all inert gases have octet and helium has duplet configuration. • Noble gases are chemically inert and will not take p ...
Diameters of rotationally and vibrationally excited diatomic molecules
... studies of transport phenomena in high-temperature gases (common in plasma processing, light sources, supersonic and hypersonic flows, etc.) if accurate molecular diameters in such gases were easily available. However, determination of the molecular diameters in high-temperature gas is much more dif ...
... studies of transport phenomena in high-temperature gases (common in plasma processing, light sources, supersonic and hypersonic flows, etc.) if accurate molecular diameters in such gases were easily available. However, determination of the molecular diameters in high-temperature gas is much more dif ...
Chapter 3 – Atomic Structure and Properties
... removed by the input of 520 kJ. The ionization process is ...
... removed by the input of 520 kJ. The ionization process is ...
CHEM 322 - Queen`s Chemistry
... Method: The course will be taught by Peter Loock, who has research interests in experimental research on electronically excited states. Each spectroscopic technique will be first introduced using fundamental QM principles, and then expanded by introducing practical applications. Evaluation: The cour ...
... Method: The course will be taught by Peter Loock, who has research interests in experimental research on electronically excited states. Each spectroscopic technique will be first introduced using fundamental QM principles, and then expanded by introducing practical applications. Evaluation: The cour ...
Name: (1 of 2) Math Set # 13 Protons, Neutrons, Electrons Proton
... An ionic bond is created between metals and nonmetals. This is because a metal in group 1 or 2 gives up electrons easily and nonmetals in groups 16 through 18 accept electrons easily. An ionic bond results in two or more ions being attracted to each other. The total charge of the molecule must be ze ...
... An ionic bond is created between metals and nonmetals. This is because a metal in group 1 or 2 gives up electrons easily and nonmetals in groups 16 through 18 accept electrons easily. An ionic bond results in two or more ions being attracted to each other. The total charge of the molecule must be ze ...
Atomic Structure Notes
... Every energy level except the first level contains three p-orbitals. Each p-orbital in the same energy level has the same energy but different orientations: x, y and z. A p-orbital in the second energy level is a 2p orbital (2px, 2py, 2pz) A p-orbital in the third energy level is a 3p orbital (3px, ...
... Every energy level except the first level contains three p-orbitals. Each p-orbital in the same energy level has the same energy but different orientations: x, y and z. A p-orbital in the second energy level is a 2p orbital (2px, 2py, 2pz) A p-orbital in the third energy level is a 3p orbital (3px, ...
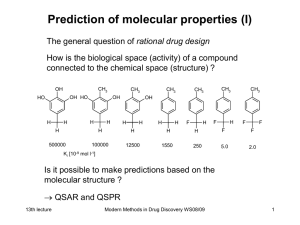
Modern Methods in Drug Discovery
... Removal of one electron ionization potential In general a disturbance by an electric field can be expressed in the form of a Taylor expansion. In the case of an external electrical field F the induced dipole moment ind is obtained as: ...
... Removal of one electron ionization potential In general a disturbance by an electric field can be expressed in the form of a Taylor expansion. In the case of an external electrical field F the induced dipole moment ind is obtained as: ...
Chemistry 1 Lectures
... Arrangement describes how the electron pairs arrange themselves around an atom ...
... Arrangement describes how the electron pairs arrange themselves around an atom ...
Molecular-scale Electronics
... shown in Figure 9-2, the conceptualization is of a single molecule between interconnecting leads that performs a signal-processing electrical function, such as rectification, resistance, etc. One can already see that many of the issues of dimensionality discussed previously should apply to such situ ...
... shown in Figure 9-2, the conceptualization is of a single molecule between interconnecting leads that performs a signal-processing electrical function, such as rectification, resistance, etc. One can already see that many of the issues of dimensionality discussed previously should apply to such situ ...
Exam Review 1: CHM 1411 Time: 0hr 55mins
... 1. The element X has three naturally occurring isotopes. The masses (amu) and % abundances of the isotopes are given in the table below. The average atomic mass of the element is ________ amu. ...
... 1. The element X has three naturally occurring isotopes. The masses (amu) and % abundances of the isotopes are given in the table below. The average atomic mass of the element is ________ amu. ...
UNIT NUM="1" ID="UN
... in the center of the field. Moreover, the electrons would be like two tiny gnats buzzing around the stadium. Atoms are mostly empty space. When two atoms approach each other during a chemical reaction, their nuclei do not come close enough to interact. Of the three kinds of subatomic particles we ha ...
... in the center of the field. Moreover, the electrons would be like two tiny gnats buzzing around the stadium. Atoms are mostly empty space. When two atoms approach each other during a chemical reaction, their nuclei do not come close enough to interact. Of the three kinds of subatomic particles we ha ...
Wave Nature of Light
... an excited state. • When the atom is in an excited state, the electron can drop from the higher-energy orbit to a lower-energy orbit. • As a result of this transition, the atom emits a photon corresponding to the difference between the energy levels associated with the two orbits. ...
... an excited state. • When the atom is in an excited state, the electron can drop from the higher-energy orbit to a lower-energy orbit. • As a result of this transition, the atom emits a photon corresponding to the difference between the energy levels associated with the two orbits. ...
Molecular orbital

In chemistry, a molecular orbital (or MO) is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term orbital was introduced by Robert S. Mulliken in 1932 as an abbreviation for one-electron orbital wave function. At an elementary level, it is used to describe the region of space in which the function has a significant amplitude. Molecular orbitals are usually constructed by combining atomic orbitals or hybrid orbitals from each atom of the molecule, or other molecular orbitals from groups of atoms. They can be quantitatively calculated using the Hartree–Fock or self-consistent field (SCF) methods.