Atomic matter of nonzero-momentum Bose-Einstein condensation and orbital current order
... Confining bosonic atoms in an optical lattice can bring out different and new physics beyond the standard BoseEinstein condensation 共BEC兲 observed in a single trap 关1,2兴. The superfluid–Mott-insulator experiment on an optical lattice 关3兴, based on an early theoretical idea 关4,5兴, demonstrated one su ...
... Confining bosonic atoms in an optical lattice can bring out different and new physics beyond the standard BoseEinstein condensation 共BEC兲 observed in a single trap 关1,2兴. The superfluid–Mott-insulator experiment on an optical lattice 关3兴, based on an early theoretical idea 关4,5兴, demonstrated one su ...
Ontological Status of Molecular Structure - HYLE-
... Abstract: Molecular structure (MS) has been treated as a convention or an epiphenomenon by physicists and quantum chemists interpreting the mathematical formalism of quantum mechanics as the essential reality criterion in the submicroscopic world (R2 world). This paper argues that, (a) even in the R ...
... Abstract: Molecular structure (MS) has been treated as a convention or an epiphenomenon by physicists and quantum chemists interpreting the mathematical formalism of quantum mechanics as the essential reality criterion in the submicroscopic world (R2 world). This paper argues that, (a) even in the R ...
An implementation of atomic form factors - IGFAE
... • n1 , l1 and m1 are integers representing the quantum numbers of the initial atomic state. • n2 , l2 and m2 are integers representing the quantum numbers of the final atomic state. • q, is a double precision argument representing the momentum transferred in the collision in atomic units: q(atomic u ...
... • n1 , l1 and m1 are integers representing the quantum numbers of the initial atomic state. • n2 , l2 and m2 are integers representing the quantum numbers of the final atomic state. • q, is a double precision argument representing the momentum transferred in the collision in atomic units: q(atomic u ...
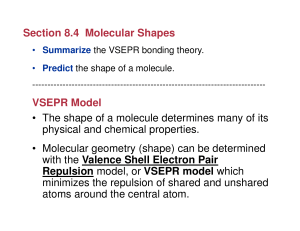
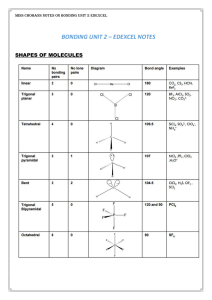
Section 8.4 Molecular Shapes VSEPR Model • The shape of a
... Shapes of Molecules (cont.) • Remember to focus on the central atom. Here is a review of the shapes: Linear- 1 bond, no central atom - 2 bonds, no unshared pairs of eBent- 2 bonds, 2 unshared pairs of ePyramidal- 3 bonds, 1 unshared pair of eTriangular planar or Trigonal3 bonds, no unshared pair of ...
... Shapes of Molecules (cont.) • Remember to focus on the central atom. Here is a review of the shapes: Linear- 1 bond, no central atom - 2 bonds, no unshared pairs of eBent- 2 bonds, 2 unshared pairs of ePyramidal- 3 bonds, 1 unshared pair of eTriangular planar or Trigonal3 bonds, no unshared pair of ...
Chapter 6.2 Notes
... ions, which form when electrons are transferred from one to another - one atom loses one or more electrons and another atom or atoms gains them - the oppositely charged ions are then attracted to each other and form an ionic bond ...
... ions, which form when electrons are transferred from one to another - one atom loses one or more electrons and another atom or atoms gains them - the oppositely charged ions are then attracted to each other and form an ionic bond ...
File
... Formation of a permanent dipole – (polar covalent) bond A polar covalent bond forms when the elements in the bond have different electronegativities . (Of around 0.3 to 1.7) When a bond is a polar covalent bond it has an unequal distribution of electrons in the bond and produces a charge separation, ...
... Formation of a permanent dipole – (polar covalent) bond A polar covalent bond forms when the elements in the bond have different electronegativities . (Of around 0.3 to 1.7) When a bond is a polar covalent bond it has an unequal distribution of electrons in the bond and produces a charge separation, ...
Conceptual Integrated Science The Elements The Periodic Table
... Copyright © 2007 Pearson Education, Inc., publishing as Pearson Addison Wesley ...
... Copyright © 2007 Pearson Education, Inc., publishing as Pearson Addison Wesley ...
Chapter 2
... • In a nonpolar covalent bond, the atoms share the electron equally • In a polar covalent bond, one atom is more electronegative, and the atoms do not share the electron equally • Unequal sharing of electrons causes a partial positive or negative charge for each atom or molecule ...
... • In a nonpolar covalent bond, the atoms share the electron equally • In a polar covalent bond, one atom is more electronegative, and the atoms do not share the electron equally • Unequal sharing of electrons causes a partial positive or negative charge for each atom or molecule ...
The Complete Notes - Joliet Junior College
... remembering. An analogy would be this: you read all the books out there on the subject of golf, but don’t get round to swinging a club – what do you think happens when you tee off for the first time? ...
... remembering. An analogy would be this: you read all the books out there on the subject of golf, but don’t get round to swinging a club – what do you think happens when you tee off for the first time? ...
Electronic state dependence in dissociation of core
... life take place in aqueous solution. The importance of water was stressed already by the ancient Greek natural philosophers. Starting with Empedocles (492-432 BC.) water was suggested to be one of the four fundamental elements in nature [1]. The theory of the four elements was the standard dogma for ...
... life take place in aqueous solution. The importance of water was stressed already by the ancient Greek natural philosophers. Starting with Empedocles (492-432 BC.) water was suggested to be one of the four fundamental elements in nature [1]. The theory of the four elements was the standard dogma for ...
Cyclo-P3 Complexes of Vanadium: Redox
... A conceptual MO diagram that highlights the most relevant molecular orbitals of the metal−cyclo-P3 interaction is shown in Figure 4. The simplest way to understand the electronic structure of 1 is to construct the key MOs from chemically meaningful molecular fragments. The most plausible building bl ...
... A conceptual MO diagram that highlights the most relevant molecular orbitals of the metal−cyclo-P3 interaction is shown in Figure 4. The simplest way to understand the electronic structure of 1 is to construct the key MOs from chemically meaningful molecular fragments. The most plausible building bl ...
VSEPR Model
... (VSEPR) model predicts the shapes of molecules and ions by assuming that the valence shell electron pairs are arranged as far from one another as possible. • To predict the relative positions of atoms around a given atom using the VSEPR model, you first note the arrangement of the electron pairs aro ...
... (VSEPR) model predicts the shapes of molecules and ions by assuming that the valence shell electron pairs are arranged as far from one another as possible. • To predict the relative positions of atoms around a given atom using the VSEPR model, you first note the arrangement of the electron pairs aro ...
A Guide to Molecular Mechanics and Quantum Chemical Calculations
... constant. Note, that the rate constant (as well as the overall rate) does not depend on the relative energies of reactants and products (“thermodynamics”) but only on the difference in energies between reactants and transition state. This difference is commonly referred to as the activation energy o ...
... constant. Note, that the rate constant (as well as the overall rate) does not depend on the relative energies of reactants and products (“thermodynamics”) but only on the difference in energies between reactants and transition state. This difference is commonly referred to as the activation energy o ...
Determination of Enzymatic Reaction Pathways Using QM/MM
... case one has not properly defined the valence bond forms (i.e., the most prevalent ionic and covalent forms), one can miss either unusual reaction pathways that can occur in reactive chemical systems or a chemical reaction not previously introduced in the valence bond forms. 2. The linear scaling app ...
... case one has not properly defined the valence bond forms (i.e., the most prevalent ionic and covalent forms), one can miss either unusual reaction pathways that can occur in reactive chemical systems or a chemical reaction not previously introduced in the valence bond forms. 2. The linear scaling app ...
Molecular orbital

In chemistry, a molecular orbital (or MO) is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term orbital was introduced by Robert S. Mulliken in 1932 as an abbreviation for one-electron orbital wave function. At an elementary level, it is used to describe the region of space in which the function has a significant amplitude. Molecular orbitals are usually constructed by combining atomic orbitals or hybrid orbitals from each atom of the molecule, or other molecular orbitals from groups of atoms. They can be quantitatively calculated using the Hartree–Fock or self-consistent field (SCF) methods.