Review redox reactions
... Iodide will react with permanganate ions to form iodine and manganese (IV) oxide. Write the balanced net ionic equation if the reaction occurs in an acidic solution. ...
... Iodide will react with permanganate ions to form iodine and manganese (IV) oxide. Write the balanced net ionic equation if the reaction occurs in an acidic solution. ...
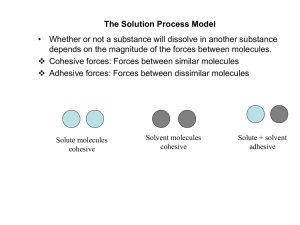
The Solution Process Model
... drug dissolves in a liquid solvent. • Dissolution is defined as the process of dissolving a solute to form a homogeneous solution as described by Noyes Whitney equation. • When a particle of a drug is dissolved in water, the molecules at the very surface of the particles dissolve and saturate the di ...
... drug dissolves in a liquid solvent. • Dissolution is defined as the process of dissolving a solute to form a homogeneous solution as described by Noyes Whitney equation. • When a particle of a drug is dissolved in water, the molecules at the very surface of the particles dissolve and saturate the di ...
www.XtremePapers.com
... Write your name, Centre number and candidate number on the Answer Sheet in the spaces provided unless this has been done for you. There are forty questions on this paper. Answer all questions. For each question there are four possible answers A, B, C and D. Choose the one you consider correct and re ...
... Write your name, Centre number and candidate number on the Answer Sheet in the spaces provided unless this has been done for you. There are forty questions on this paper. Answer all questions. For each question there are four possible answers A, B, C and D. Choose the one you consider correct and re ...
A Review of High School Chemistry
... But valuable also means boring. If you want real fun, you hang out with active metals like potassium and magnesium and lithium, active metals that explode at the slightest provocation (like placing them in water.) So what does happen when you throw a metal in water? M + H2O Æ M+ + H2 + OH• The metal ...
... But valuable also means boring. If you want real fun, you hang out with active metals like potassium and magnesium and lithium, active metals that explode at the slightest provocation (like placing them in water.) So what does happen when you throw a metal in water? M + H2O Æ M+ + H2 + OH• The metal ...
Handout on Buffer Solutions
... “Self, why don’t I just plug the concentration of NaA and HA into the H-H equation and solve for pH? Why do all this work?” Although the H-H equation is always valid, the [A-] and [HA] in the H-H equation are the equilibrium concentrations. Once you know [A-] and [HA] at equilibrium, you can plug th ...
... “Self, why don’t I just plug the concentration of NaA and HA into the H-H equation and solve for pH? Why do all this work?” Although the H-H equation is always valid, the [A-] and [HA] in the H-H equation are the equilibrium concentrations. Once you know [A-] and [HA] at equilibrium, you can plug th ...
E - Purdue Physics
... Electrons are not completely free – they are bound to the metal as a whole. We will return to this idea when we discuss the force on a current carrying wire in a magnetic field. There is no net interaction between mobile electrons ...
... Electrons are not completely free – they are bound to the metal as a whole. We will return to this idea when we discuss the force on a current carrying wire in a magnetic field. There is no net interaction between mobile electrons ...
Net ionic equation
... also called exchange or metathesis A solid (precipitate) forms in these reactions ??How do we know what the precipitate will be?? ...
... also called exchange or metathesis A solid (precipitate) forms in these reactions ??How do we know what the precipitate will be?? ...
Bellin College Homework Supplement
... lowering the body temperature will reduce the amount of oxygen needed by the body. Some methods used to lower body temperature include cooled saline solution, cool water blankets, or cooling caps worn on the head. How many kilojoules are lost when the body temperature of a surgery patient with a blo ...
... lowering the body temperature will reduce the amount of oxygen needed by the body. Some methods used to lower body temperature include cooled saline solution, cool water blankets, or cooling caps worn on the head. How many kilojoules are lost when the body temperature of a surgery patient with a blo ...
Lecture 4
... Important exceptions are acids and compounds such as ammonia that react with water to form ions. Strong and Weak Electrolytes Strong electrolytes exist in solution completely or nearly completely as ions. Weak electrolytes produce small concentrations of ions when they dissolve. Do not confuse the e ...
... Important exceptions are acids and compounds such as ammonia that react with water to form ions. Strong and Weak Electrolytes Strong electrolytes exist in solution completely or nearly completely as ions. Weak electrolytes produce small concentrations of ions when they dissolve. Do not confuse the e ...
Equilibrium chemistry
Equilibrium chemistry is a concerned with systems in chemical equilibrium. The unifying principle is that the free energy of a system at equilibrium is the minimum possible, so that the slope of the free energy with respect to the reaction coordinate is zero. This principle, applied to mixtures at equilibrium provides a definition of an equilibrium constant. Applications include acid-base, host-guest, metal-complex, solubility, partition, chromatography and redox equilibria.