Hydroxyl Group of a Phosphorylated Ribose
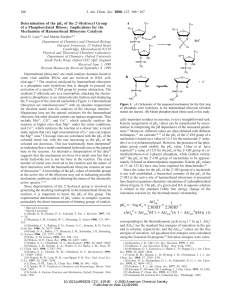
... that readily catalyze the reaction. It therefore seems unlikely that metal hydroxide ions act as the general base in the reaction.10 Since a metal ion is known to bind directly to the adjacent pro-R oxygen of the phosphate, it is conceivable that it promotes the reaction by stabilizing the transitio ...
... that readily catalyze the reaction. It therefore seems unlikely that metal hydroxide ions act as the general base in the reaction.10 Since a metal ion is known to bind directly to the adjacent pro-R oxygen of the phosphate, it is conceivable that it promotes the reaction by stabilizing the transitio ...
Questions - Chemistry Teaching Resources
... This mixture contains quinone, C6H4O2, a compound that is formed by the reaction of hydroquinone, C6H4(OH)2, with hydrogen peroxide, H2O2. The reaction is catalysed by an enzyme called catalase and the equation for the overall reaction is: ...
... This mixture contains quinone, C6H4O2, a compound that is formed by the reaction of hydroquinone, C6H4(OH)2, with hydrogen peroxide, H2O2. The reaction is catalysed by an enzyme called catalase and the equation for the overall reaction is: ...
ap chemistry syllabus
... Enduring Understanding 5.B-Energy is neither created nor destroyed, but only transformed from one form to another. Enduring Understanding 5.C-Breaking bonds requires energy, and making bonds releases energy. Enduring Understanding 5.D-Electrostatic forces exist between molecules as well as between a ...
... Enduring Understanding 5.B-Energy is neither created nor destroyed, but only transformed from one form to another. Enduring Understanding 5.C-Breaking bonds requires energy, and making bonds releases energy. Enduring Understanding 5.D-Electrostatic forces exist between molecules as well as between a ...
chemistry-subject test5 w. solutions
... and 2.07 moles of CO exert a total pressure of 234 torr, then the partial pressure of CO is given by: ...
... and 2.07 moles of CO exert a total pressure of 234 torr, then the partial pressure of CO is given by: ...
Polysulfane Antitumor Agents from o
... Information). These results provide evidence for the formation of 5 in the benzyne-Sx reaction and are consistent with the idea that heat or nucleophiles can influence the equilibrium between polysulfane compounds,7a,11-13 which offers a reason for the decomposition of o-C6H4S8 5 under the condition ...
... Information). These results provide evidence for the formation of 5 in the benzyne-Sx reaction and are consistent with the idea that heat or nucleophiles can influence the equilibrium between polysulfane compounds,7a,11-13 which offers a reason for the decomposition of o-C6H4S8 5 under the condition ...
Organised Surfactant System: Micro Emulsion
... adsorbed monolayer to spontaneously achieve zero interfacial tension. This causes the droplets to break up spontaneously ensuring that the system will remain dispersed & will not, as microemulsions do, achieve equilibrium by separating into the original, mutually insoluble liquid phase’s viz. phase ...
... adsorbed monolayer to spontaneously achieve zero interfacial tension. This causes the droplets to break up spontaneously ensuring that the system will remain dispersed & will not, as microemulsions do, achieve equilibrium by separating into the original, mutually insoluble liquid phase’s viz. phase ...
Thermodynamics: Entropy and Free Energy
... + oxygen gas. Energy is conserved whether the process runs backward or forward, but there is an allowed direction in which these events always occur. In fact, most chemical and physical changes naturally occur in one direction and can occur in the opposite direction only with assistance. For example ...
... + oxygen gas. Energy is conserved whether the process runs backward or forward, but there is an allowed direction in which these events always occur. In fact, most chemical and physical changes naturally occur in one direction and can occur in the opposite direction only with assistance. For example ...
The hydrogen bonding of cytosine with guanine
... Chemical Labomtmy, Duke U n i s i t y , Durham, North Carolina 27706 synopsis The enthalpy of hydrogen-bond formation between guanine (G) and cytusine (C) in o-dichlorobenzene and in chloroform at 25°C has been determined by direct calorimetric measurement. We derivatized 2’-deoxyguanosine and 2‘-de ...
... Chemical Labomtmy, Duke U n i s i t y , Durham, North Carolina 27706 synopsis The enthalpy of hydrogen-bond formation between guanine (G) and cytusine (C) in o-dichlorobenzene and in chloroform at 25°C has been determined by direct calorimetric measurement. We derivatized 2’-deoxyguanosine and 2‘-de ...
Chapter 18.2
... Equations [19.1] • (Note: This section is usually covered with a very brief review of oxidation numbers and oxidizing/reducing agents, as covered in Chemistry 1010, which is found in section 4.9 of the Tro text). ...
... Equations [19.1] • (Note: This section is usually covered with a very brief review of oxidation numbers and oxidizing/reducing agents, as covered in Chemistry 1010, which is found in section 4.9 of the Tro text). ...
Chapter 20 Thermodynamics
... is the same even though they took very different paths to get to the mountain top. This is the power of all the state functions in thermodynamics---we only need to now the start and the finish bulk parameters. ...
... is the same even though they took very different paths to get to the mountain top. This is the power of all the state functions in thermodynamics---we only need to now the start and the finish bulk parameters. ...
Topic guide 6.2: Phase equilibria
... conditions of pressure and temperature at which four different phases can be in equilibrium. This is predicted by the phase rule; this predicts the number of degrees of freedom that can exist for a system at equilibrium. The number of degrees of freedom is the number of physical variables that can b ...
... conditions of pressure and temperature at which four different phases can be in equilibrium. This is predicted by the phase rule; this predicts the number of degrees of freedom that can exist for a system at equilibrium. The number of degrees of freedom is the number of physical variables that can b ...
Role of Chemical Reaction Engineering in Sustainable
... selecting and developing the right catalyst has a huge impact on the success of a proposed process route. However, it may be noted that the development of novel catalyst has to be often combined with novel reactor technology for the process to be economically viable and environmentally beneficial. H ...
... selecting and developing the right catalyst has a huge impact on the success of a proposed process route. However, it may be noted that the development of novel catalyst has to be often combined with novel reactor technology for the process to be economically viable and environmentally beneficial. H ...
Maximum Caliber: A variational approach applied to two
... where Qd = 兺 jexp兵1A1j + ¯ + LALj其 denotes the dynamical partition function. In complete analogy to MaxEnt, by maximizing Eq. 共1.7兲, we select the path distribution 兵pⴱj 共t兲其 with the greatest multiplicity that agrees with the given information 共1.2兲 and 共1.6兲. This path distribution then determin ...
... where Qd = 兺 jexp兵1A1j + ¯ + LALj其 denotes the dynamical partition function. In complete analogy to MaxEnt, by maximizing Eq. 共1.7兲, we select the path distribution 兵pⴱj 共t兲其 with the greatest multiplicity that agrees with the given information 共1.2兲 and 共1.6兲. This path distribution then determin ...
The Major Classes of Chemical Reactions
... a much more active role. It interacts strongly with the reactants and, in some cases, even affects their bonds. Water is crucial to many chemical and physical processes, and we will discuss its properties frequently in the text. The Solubility of Ionic Compounds When water dissolves an ionic solid, ...
... a much more active role. It interacts strongly with the reactants and, in some cases, even affects their bonds. Water is crucial to many chemical and physical processes, and we will discuss its properties frequently in the text. The Solubility of Ionic Compounds When water dissolves an ionic solid, ...
Equilibrium chemistry
Equilibrium chemistry is a concerned with systems in chemical equilibrium. The unifying principle is that the free energy of a system at equilibrium is the minimum possible, so that the slope of the free energy with respect to the reaction coordinate is zero. This principle, applied to mixtures at equilibrium provides a definition of an equilibrium constant. Applications include acid-base, host-guest, metal-complex, solubility, partition, chromatography and redox equilibria.























