thermodynamics
... quantitatively each of the properties such as its pressure (p), volume (V), and temperature (T ) as well as the composition of the system. We need to describe the system by specifying it before and after the change. You would recall from your Physics course that the state of a system in mechanics is ...
... quantitatively each of the properties such as its pressure (p), volume (V), and temperature (T ) as well as the composition of the system. We need to describe the system by specifying it before and after the change. You would recall from your Physics course that the state of a system in mechanics is ...
precipitate - UniMAP Portal
... For a successful determination in gravimetric analysis the following criteria should be met :(1)The desired substance must be completely precipitated. In most determination the precipitate is of such low solubility that losses from dissolution are negligible. An additional factor is the common ion ...
... For a successful determination in gravimetric analysis the following criteria should be met :(1)The desired substance must be completely precipitated. In most determination the precipitate is of such low solubility that losses from dissolution are negligible. An additional factor is the common ion ...
Thermodynamics: the Second Law
... No process is possible in which the sole result is the absorption of heat from a reservoir and its complete conversion to work (formulated by Kelvin). It has proved impossible to construct an engine, in which heat is drawn from a hot reservoir and completely converted into work. All real engines hav ...
... No process is possible in which the sole result is the absorption of heat from a reservoir and its complete conversion to work (formulated by Kelvin). It has proved impossible to construct an engine, in which heat is drawn from a hot reservoir and completely converted into work. All real engines hav ...
Hydrolysis of Phytic Acid by Microwave Treatment: Application to
... determination in this work has the advantage that after 45 min, the final absorbance is not dependent on the nature of the acid used and its concentration. Nevertheless, the kinetics of heteropoly acid formation was affected by acidity conditions. In general, the higher the acid concentration, the f ...
... determination in this work has the advantage that after 45 min, the final absorbance is not dependent on the nature of the acid used and its concentration. Nevertheless, the kinetics of heteropoly acid formation was affected by acidity conditions. In general, the higher the acid concentration, the f ...
File
... A pure aluminium block with a mass of 10 g is heated so that its temperature increases from 20 °C to 50 °C . The specific heat capacity of aluminium is 8.99 × 10–1 J g–1 K–1. Which expression gives the heat energy change in kJ? A. ...
... A pure aluminium block with a mass of 10 g is heated so that its temperature increases from 20 °C to 50 °C . The specific heat capacity of aluminium is 8.99 × 10–1 J g–1 K–1. Which expression gives the heat energy change in kJ? A. ...
Document
... Since the solutions we have to compare are all aqueous (ie solvent = water) and that their molality is identical, the comparison of their respective i is sufficient. The ideal (or limiting) values of i for each electrolyte are tabulated below: ...
... Since the solutions we have to compare are all aqueous (ie solvent = water) and that their molality is identical, the comparison of their respective i is sufficient. The ideal (or limiting) values of i for each electrolyte are tabulated below: ...
Kinetic study on carbonation of crude Li2CO3 with CO2
... provide relatively low-cost feedstock to prepare high purity Li2CO3. The work aimed at purifying Li2CO3 from such feedstock via its slurry phase dissolution in a slurry bubble column reactor. Commercial crude Li2CO3 produced from lithium-containing minerals, brines or sea water carries considerable ...
... provide relatively low-cost feedstock to prepare high purity Li2CO3. The work aimed at purifying Li2CO3 from such feedstock via its slurry phase dissolution in a slurry bubble column reactor. Commercial crude Li2CO3 produced from lithium-containing minerals, brines or sea water carries considerable ...
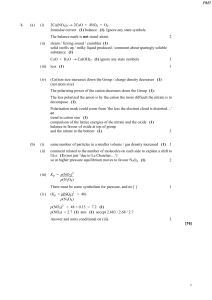
1. (a) (i) 2Ca(NO3)2 → 2CaO + 4NO2 + O2 formulae correct (1
... and that of HCl are both complete (1) that the second ionisation of sulphuric is suppressed by the H+ from the first (1) little contribution from 2nd ionisation so reduces the pH very little / increases the [H+] very little (1) ...
... and that of HCl are both complete (1) that the second ionisation of sulphuric is suppressed by the H+ from the first (1) little contribution from 2nd ionisation so reduces the pH very little / increases the [H+] very little (1) ...
Downloaded from www.studiestoday.com Downloaded from www
... Solution 16. The α -form of glucose and β -form of glucose can be distinguished by the position of the hydroxyl group on the first carbon atom. In open chain α -glucose, the hydroxyl group on the first carbon atom is towards the right whereas, in the closed ring α - glucose, the hydroxyl group on th ...
... Solution 16. The α -form of glucose and β -form of glucose can be distinguished by the position of the hydroxyl group on the first carbon atom. In open chain α -glucose, the hydroxyl group on the first carbon atom is towards the right whereas, in the closed ring α - glucose, the hydroxyl group on th ...
Stoichiometry of Chemical Reactions
... Shuttle fleet (Figure 4.1). The engines of these rockets rely on carefully prepared solid mixtures of chemicals combined in precisely measured amounts. Igniting the mixture initiates a vigorous chemical reaction that rapidly generates large amounts of gaseous products. These gases are ejected from t ...
... Shuttle fleet (Figure 4.1). The engines of these rockets rely on carefully prepared solid mixtures of chemicals combined in precisely measured amounts. Igniting the mixture initiates a vigorous chemical reaction that rapidly generates large amounts of gaseous products. These gases are ejected from t ...
Genericity of confined chemical garden patterns with regard to
... CoCl2 solutions used in the reference diagram (Fig. 2A) varies by two units within the range of concentrations used. Since the precipitation reaction could be influenced by both the pH and concentration of reactants, we need to disentangle these two effects on the pattern selection. For this purpose, ...
... CoCl2 solutions used in the reference diagram (Fig. 2A) varies by two units within the range of concentrations used. Since the precipitation reaction could be influenced by both the pH and concentration of reactants, we need to disentangle these two effects on the pattern selection. For this purpose, ...
Equilibrium chemistry
Equilibrium chemistry is a concerned with systems in chemical equilibrium. The unifying principle is that the free energy of a system at equilibrium is the minimum possible, so that the slope of the free energy with respect to the reaction coordinate is zero. This principle, applied to mixtures at equilibrium provides a definition of an equilibrium constant. Applications include acid-base, host-guest, metal-complex, solubility, partition, chromatography and redox equilibria.