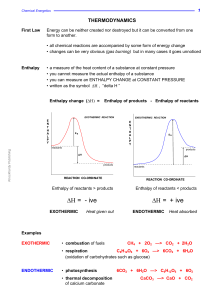
High-Pressure Solubility Data of Methane in Aniline
... The application of high pressures to industrial processes has led to engineering operations that frequently require knowledge of solubilities of gases in liquids at pressures higher than those for which such data are ordinarily available. The production of aniline by reduction of nitrobenzene is suc ...
... The application of high pressures to industrial processes has led to engineering operations that frequently require knowledge of solubilities of gases in liquids at pressures higher than those for which such data are ordinarily available. The production of aniline by reduction of nitrobenzene is suc ...
Stoichiometry Notes
... This is a method in which a substance is taken in excess and some part of it has to react with another substance and the remaining part has to be titrated against standard reagent. Double titration : This is a titration of specific compound using different indicators. Let us consider a solid mixture ...
... This is a method in which a substance is taken in excess and some part of it has to react with another substance and the remaining part has to be titrated against standard reagent. Double titration : This is a titration of specific compound using different indicators. Let us consider a solid mixture ...
MALTA
... differential equation, boundary conditions. 3. Calculus (Applications): Application of differentiation to locate and identify turning points. The role of calculus in thermodynamics. The use of integration to calculate p-V work (i.e. to find the area under p-V graphs). Integration as a means to obtai ...
... differential equation, boundary conditions. 3. Calculus (Applications): Application of differentiation to locate and identify turning points. The role of calculus in thermodynamics. The use of integration to calculate p-V work (i.e. to find the area under p-V graphs). Integration as a means to obtai ...
(MgCl2 and CaCl2): Osmotic Pressure Calculations
... LJ parameters of the dummy atoms remain the same among different ions, the LJ parameters of the central atom differ and are based on their respective match to the hydration free energy. The starting parameter values of the model are shown in Table 1.43 ...
... LJ parameters of the dummy atoms remain the same among different ions, the LJ parameters of the central atom differ and are based on their respective match to the hydration free energy. The starting parameter values of the model are shown in Table 1.43 ...
Role of Substrate Temperature on the Structural
... In this study, ZnO thin films have been prepared by USP technique, which involves spraying a zinc acetate solution onto a heated substrate. Zinc acetate (Zn(CH3 COO)2 ) was first suggested as a potential single-source precursor by Tammenmaa et al [5] for ZnO film growth by atomic layer epitaxy. Highly ...
... In this study, ZnO thin films have been prepared by USP technique, which involves spraying a zinc acetate solution onto a heated substrate. Zinc acetate (Zn(CH3 COO)2 ) was first suggested as a potential single-source precursor by Tammenmaa et al [5] for ZnO film growth by atomic layer epitaxy. Highly ...
Theory of Ion Exchange
... side of eqn [12] constant), the ratio EA/EB must decrease as CT is decreased when zA'zB. Thus, the relative concentration of ion A must decrease with decreasing CT. This feature, the increased preference of an ion exchanger for the ion having a higher charge with the dilution of the solution, is cal ...
... side of eqn [12] constant), the ratio EA/EB must decrease as CT is decreased when zA'zB. Thus, the relative concentration of ion A must decrease with decreasing CT. This feature, the increased preference of an ion exchanger for the ion having a higher charge with the dilution of the solution, is cal ...
Advanced Kinetic Analysis Using a LAMBDA Series Spectrometer
... An accurate enzyme assay requires the proper control of pH and temperature, usually a small volume of sample (often only between 10 and 100 µL), and the use of optimized data collection interval for the concentration range being studied: high reaction rates require smaller data intervals on the time ...
... An accurate enzyme assay requires the proper control of pH and temperature, usually a small volume of sample (often only between 10 and 100 µL), and the use of optimized data collection interval for the concentration range being studied: high reaction rates require smaller data intervals on the time ...
PAGE PROOFS
... 1. Write both full and ionic equations for the reactions that result when zinc and HCl are mixed. Assume the reactions go to completion. 2. Write both full and ionic equations for the reactions that result when the following substances are mixed. Assume all reactions go to completion. (a) magnesi ...
... 1. Write both full and ionic equations for the reactions that result when zinc and HCl are mixed. Assume the reactions go to completion. 2. Write both full and ionic equations for the reactions that result when the following substances are mixed. Assume all reactions go to completion. (a) magnesi ...
PX312-1718
... 44. The reaction CaO(s) + SO3(g) CaSO4(s) is nonspontaneous at 2200 K, whereas it is spontaneous at room temperature. Which of the following statements is false? A) The change in enthalpy is the main driving force of the reaction. B) Both H and S are negative for the reaction. C) G is negative ...
... 44. The reaction CaO(s) + SO3(g) CaSO4(s) is nonspontaneous at 2200 K, whereas it is spontaneous at room temperature. Which of the following statements is false? A) The change in enthalpy is the main driving force of the reaction. B) Both H and S are negative for the reaction. C) G is negative ...
Equilibrium chemistry
Equilibrium chemistry is a concerned with systems in chemical equilibrium. The unifying principle is that the free energy of a system at equilibrium is the minimum possible, so that the slope of the free energy with respect to the reaction coordinate is zero. This principle, applied to mixtures at equilibrium provides a definition of an equilibrium constant. Applications include acid-base, host-guest, metal-complex, solubility, partition, chromatography and redox equilibria.