Chapter 11 * Chemical Reactions
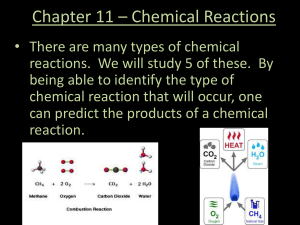
... • There are many types of chemical reactions. We will study 5 of these. By being able to identify the type of chemical reaction that will occur, one can predict the products of a chemical reaction. ...
... • There are many types of chemical reactions. We will study 5 of these. By being able to identify the type of chemical reaction that will occur, one can predict the products of a chemical reaction. ...
The Effect of Temperature on Spontaneity
... increases the random motions and the entropy of the surroundings. ΔSsurr is positive The magnitude of ΔSsurr depends on the temperature. The transfer of a given quantity of energy as a heat produces much greater percent change in the randomness of the surrounding at a low temperature than it does at ...
... increases the random motions and the entropy of the surroundings. ΔSsurr is positive The magnitude of ΔSsurr depends on the temperature. The transfer of a given quantity of energy as a heat produces much greater percent change in the randomness of the surrounding at a low temperature than it does at ...
Spring Exam 2 - Chemistry
... It is extremely important that you fill in the answer sheet EXACTLY as indicated, otherwise your answer sheet may not be processed; ALL entries are to be made on SIDE 1 of the answer sheet. Use a #2 pencil (or softer); fill in the circles completely and firmly. Erasures must be complete. Use only th ...
... It is extremely important that you fill in the answer sheet EXACTLY as indicated, otherwise your answer sheet may not be processed; ALL entries are to be made on SIDE 1 of the answer sheet. Use a #2 pencil (or softer); fill in the circles completely and firmly. Erasures must be complete. Use only th ...
Presentation
... Balancing Chemical Equations Concept Check Which of the following are true concerning balanced chemical equations? There may be more than one true statement. I. The number of molecules is conserved. II. The coefficients tell you how much of each ...
... Balancing Chemical Equations Concept Check Which of the following are true concerning balanced chemical equations? There may be more than one true statement. I. The number of molecules is conserved. II. The coefficients tell you how much of each ...
A) I is TRUE, II is FALSE B) I is FALSE, II is TRUE C) I and II
... diethyl ether, CH3CH 2OCH2CH 3 at 20° C, to gaseous diethyl ether at its boiling point, 30 ° C? A) 2.30 kJ C) 37.5 kJ E) 39.8 kJ ...
... diethyl ether, CH3CH 2OCH2CH 3 at 20° C, to gaseous diethyl ether at its boiling point, 30 ° C? A) 2.30 kJ C) 37.5 kJ E) 39.8 kJ ...
Sherbert
... Make some predictions about what will happen before you observe. Make some observations and write them down. Try and explain your observations and how the observations are connected. Remember, a prediction in science is a testable hypothesis. You must make a statement about what you think will hap ...
... Make some predictions about what will happen before you observe. Make some observations and write them down. Try and explain your observations and how the observations are connected. Remember, a prediction in science is a testable hypothesis. You must make a statement about what you think will hap ...
Chapter 6
... A student carries out an experiment to standardize a sodium hydroxide solution. To do this, the student weighs out 1.3009 g sample of potassium hydrogen phthalate (KHC8H4O4 or KHP–molar mass 204.22 g/mol). The student dissolves the KHP in distilled water, adds phenolphthalein as an indicator, and ti ...
... A student carries out an experiment to standardize a sodium hydroxide solution. To do this, the student weighs out 1.3009 g sample of potassium hydrogen phthalate (KHC8H4O4 or KHP–molar mass 204.22 g/mol). The student dissolves the KHP in distilled water, adds phenolphthalein as an indicator, and ti ...
Selenium dioxide catalysed oxidation of acetic acid hydrazide by
... of 27.0 + 0.1◦ C. The reaction was initiated by mixing the previously thermostated solutions of the oxidant, catalyst and substrate, which also contained the required amount of hydrochloric acid, sodium perchlorate and distilled water. The reaction was followed by titrating 5 cm3 of the reaction mix ...
... of 27.0 + 0.1◦ C. The reaction was initiated by mixing the previously thermostated solutions of the oxidant, catalyst and substrate, which also contained the required amount of hydrochloric acid, sodium perchlorate and distilled water. The reaction was followed by titrating 5 cm3 of the reaction mix ...
chapter 1 - Revsworld
... The contribution for which de Broglie is remembered in modern science is a) his statement that an electron can exist in an atom in discrete energy levels. b) his statement that no electron can have identical values for all 4 quantum numbers. c) his statement that electrons occupy all the orbitals of ...
... The contribution for which de Broglie is remembered in modern science is a) his statement that an electron can exist in an atom in discrete energy levels. b) his statement that no electron can have identical values for all 4 quantum numbers. c) his statement that electrons occupy all the orbitals of ...
Microwave-Specific Enhancement of the Carbon−Carbon Dioxide
... the thermodynamic parameters, specifically ΔH and ΔS, were calculated using standard thermodynamic tables. The enthalpy and entropy were corrected for temperature using the heat capacities of the reactants and products to better match the temperatures at which the microwave reaction was experimentall ...
... the thermodynamic parameters, specifically ΔH and ΔS, were calculated using standard thermodynamic tables. The enthalpy and entropy were corrected for temperature using the heat capacities of the reactants and products to better match the temperatures at which the microwave reaction was experimentall ...
Synthesis Reactions occur when two of more reactants combine to
... Use 3 drops of solution, just enough to cover the piece of metal. Do not perform a reaction of a metal with its own solution (ex. copper metal and CuCl2 solution)!! This creates unnecessary chemical waste. DATA: Create a data table for observations of the reaction of each metal with each solution. E ...
... Use 3 drops of solution, just enough to cover the piece of metal. Do not perform a reaction of a metal with its own solution (ex. copper metal and CuCl2 solution)!! This creates unnecessary chemical waste. DATA: Create a data table for observations of the reaction of each metal with each solution. E ...
Transition state theory
Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ‡Hɵ), the standard entropy of activation (Δ‡Sɵ), and the standard Gibbs energy of activation (Δ‡Gɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest at the transition state.)This theory was developed simultaneously in 1935 by Henry Eyring, then at Princeton University, and by Meredith Gwynne Evans and Michael Polanyi of the University of Manchester. TST is also referred to as ""activated-complex theory,"" ""absolute-rate theory,"" and ""theory of absolute reaction rates.""Before the development of TST, the Arrhenius rate law was widely used to determine energies for the reaction barrier. The Arrhenius equation derives from empirical observations and ignores any mechanistic considerations, such as whether one or more reactive intermediates are involved in the conversion of a reactant to a product. Therefore, further development was necessary to understand the two parameters associated with this law, the pre-exponential factor (A) and the activation energy (Ea). TST, which led to the Eyring equation, successfully addresses these two issues; however, 46 years elapsed between the publication of the Arrhenius rate law, in 1889, and the Eyring equation derived from TST, in 1935. During that period, many scientists and researchers contributed significantly to the development of the theory.