Chemical Thermodynamics -
... E = E - [RT / nF] ln Q The Nernst Equation shows the relationship between the standard cell potential ( E ) and the cell potential ( E ) under actual, non-standard conditions. this can be simplified at 25C to: ...
... E = E - [RT / nF] ln Q The Nernst Equation shows the relationship between the standard cell potential ( E ) and the cell potential ( E ) under actual, non-standard conditions. this can be simplified at 25C to: ...
Net Ionic Equations
... Steps in writing a net ionic equation: • Write the conventional equation, including designations of state [(g), (l), (s), (aq)]. Balance the equation. • Write the ionic equation by replacing each dissolved substance (aq) with its solution inventory species. Never change states in this step. Be sure ...
... Steps in writing a net ionic equation: • Write the conventional equation, including designations of state [(g), (l), (s), (aq)]. Balance the equation. • Write the ionic equation by replacing each dissolved substance (aq) with its solution inventory species. Never change states in this step. Be sure ...
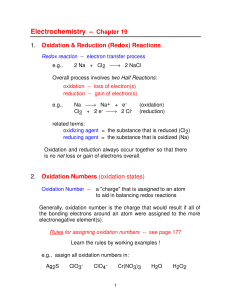
Balancing reaction equations, oxidation state, and reduction
... Example: H2 combining with O2 to form water: 2 H2 + O2 → 2H2O An electron is transferred from H to O: the H2 is oxidized and the O2 is reduced. We use the oxidation number (oxidation state) to keep track of electron shifts in chemical reactions. It is defined as “the charge which an atom appears to ...
... Example: H2 combining with O2 to form water: 2 H2 + O2 → 2H2O An electron is transferred from H to O: the H2 is oxidized and the O2 is reduced. We use the oxidation number (oxidation state) to keep track of electron shifts in chemical reactions. It is defined as “the charge which an atom appears to ...
Document
... E = E° - [RT / nF] ln Q The Nernst Equation shows the relationship between the standard cell potential ( E° ) and the cell potential ( E ) under actual, non-standard conditions. this can be simplified at 25°C to: ...
... E = E° - [RT / nF] ln Q The Nernst Equation shows the relationship between the standard cell potential ( E° ) and the cell potential ( E ) under actual, non-standard conditions. this can be simplified at 25°C to: ...
North Carolina Test of Chemistry RELEASED
... © 2009 All rights reserved. This document may not be reproduced by any means, in whole or in part, without prior written permission from the North Carolina Department of Public Instruction, Raleigh, North Carolina. ...
... © 2009 All rights reserved. This document may not be reproduced by any means, in whole or in part, without prior written permission from the North Carolina Department of Public Instruction, Raleigh, North Carolina. ...
Final "I Can Statements" Answer Key
... are theoretical although some gases are close. Hydrogen and helium are the closest to ideal gases at all temperatures and pressures. A gas will act most “ideally” under the conditions of __low___ pressure and ___high_____ temperature. The two elements that act ideally most of the time are ___hydroge ...
... are theoretical although some gases are close. Hydrogen and helium are the closest to ideal gases at all temperatures and pressures. A gas will act most “ideally” under the conditions of __low___ pressure and ___high_____ temperature. The two elements that act ideally most of the time are ___hydroge ...
chemistry 110 lecture
... Keys: 1. Know the physical states of the elements (g) (l) (s) (aq) 2. Know solubility rules 3. Balancing equations a) Count and compare the number of atoms of each element on both sides of the equation. b) Balance each element individually by placing whole numbers in front of the chemical formula c) ...
... Keys: 1. Know the physical states of the elements (g) (l) (s) (aq) 2. Know solubility rules 3. Balancing equations a) Count and compare the number of atoms of each element on both sides of the equation. b) Balance each element individually by placing whole numbers in front of the chemical formula c) ...
H 2 (g)
... Specific heat capacity, c of a substance is the amount of heat required to raise the temperature of one gram of the substance by one degree Celsius (Jg 1C1). Heat capacity, C Heat capacity,C is the amount of heat required to raise the temperature of a given quantity of the substance by one de ...
... Specific heat capacity, c of a substance is the amount of heat required to raise the temperature of one gram of the substance by one degree Celsius (Jg 1C1). Heat capacity, C Heat capacity,C is the amount of heat required to raise the temperature of a given quantity of the substance by one de ...
Unit 1 Matter Day 32 2016 Counting Atoms
... *Because they require that you have the same mass after the reaction as you do before the reaction. This means… *The # and type of atoms are the same in the reactants and products (just in different combinations) *Although the state of matter of the products may be different from the reactants, the ...
... *Because they require that you have the same mass after the reaction as you do before the reaction. This means… *The # and type of atoms are the same in the reactants and products (just in different combinations) *Although the state of matter of the products may be different from the reactants, the ...
Chemical Equations - Salem Community Schools
... around you all the time. • Many important clues indicate when chemical reactions occur. • None of them alone proves that such a change occurs because some physical changes involve one or more of these signs. ...
... around you all the time. • Many important clues indicate when chemical reactions occur. • None of them alone proves that such a change occurs because some physical changes involve one or more of these signs. ...
UNIT 5 - H-W Science Website
... LAB: Bond Breaking and the Heat of Reaction All chemical reactions take place with either an absorption or release of energy. Generally this energy is in the form of heat, but in some processes it may take the form of mainly light, or a mixture of forms including some mechanical energy such as soun ...
... LAB: Bond Breaking and the Heat of Reaction All chemical reactions take place with either an absorption or release of energy. Generally this energy is in the form of heat, but in some processes it may take the form of mainly light, or a mixture of forms including some mechanical energy such as soun ...
Matter - Wsfcs
... KoolAid and then a glass of water with a little milk mixed in, which one(s) would you see the light beam going through the ...
... KoolAid and then a glass of water with a little milk mixed in, which one(s) would you see the light beam going through the ...
CBSE/12th Class/2010/CHEMISTRY
... Ionic solids Ionic solids are insulators in solid state but conductors in molten state and in aqueous solutions. Ans.2 In chemical kinetics, the order of reaction with respect to a given substance (such as reactant, catalyst or product) is defined as the index, or exponent, to which its concentratio ...
... Ionic solids Ionic solids are insulators in solid state but conductors in molten state and in aqueous solutions. Ans.2 In chemical kinetics, the order of reaction with respect to a given substance (such as reactant, catalyst or product) is defined as the index, or exponent, to which its concentratio ...
8 Chemical Equations Chapter Outline Chemical Equations
... Write the balanced chemical equation for the reaction of magnesium hydroxide and phosphoric acid to form magnesium phosphate and water. a. 3 Mg(OH)2 + 2 H3PO4 b. Mg(OH)2 + H3PO4 ...
... Write the balanced chemical equation for the reaction of magnesium hydroxide and phosphoric acid to form magnesium phosphate and water. a. 3 Mg(OH)2 + 2 H3PO4 b. Mg(OH)2 + H3PO4 ...
Transition state theory
Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ‡Hɵ), the standard entropy of activation (Δ‡Sɵ), and the standard Gibbs energy of activation (Δ‡Gɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest at the transition state.)This theory was developed simultaneously in 1935 by Henry Eyring, then at Princeton University, and by Meredith Gwynne Evans and Michael Polanyi of the University of Manchester. TST is also referred to as ""activated-complex theory,"" ""absolute-rate theory,"" and ""theory of absolute reaction rates.""Before the development of TST, the Arrhenius rate law was widely used to determine energies for the reaction barrier. The Arrhenius equation derives from empirical observations and ignores any mechanistic considerations, such as whether one or more reactive intermediates are involved in the conversion of a reactant to a product. Therefore, further development was necessary to understand the two parameters associated with this law, the pre-exponential factor (A) and the activation energy (Ea). TST, which led to the Eyring equation, successfully addresses these two issues; however, 46 years elapsed between the publication of the Arrhenius rate law, in 1889, and the Eyring equation derived from TST, in 1935. During that period, many scientists and researchers contributed significantly to the development of the theory.