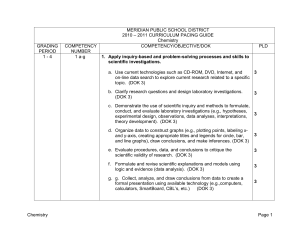
Complete the following equations
... The Oswald process for the production of nitric acid involves the following reactions: (i) 4NH3(g) + 5 O2(g) 4 NO(g) + 6H2O(g); (ii) 2 NO(g) + O2(g) 2 NO2(g); (iii) 3 NO2(g) + H2O(l) 2 HNO3(l) + NO(g); (a) Calculate the enthalpy change (H, in kJ) for each reaction. (b) Balance the following e ...
... The Oswald process for the production of nitric acid involves the following reactions: (i) 4NH3(g) + 5 O2(g) 4 NO(g) + 6H2O(g); (ii) 2 NO(g) + O2(g) 2 NO2(g); (iii) 3 NO2(g) + H2O(l) 2 HNO3(l) + NO(g); (a) Calculate the enthalpy change (H, in kJ) for each reaction. (b) Balance the following e ...
Document
... The glass transition A melt eventually transforms to a glass if crystallization is by-passed upon cooling. This transition from a liquid to a solid is accompanied by marked changes in second-order thermodynamic properties. To account for these changes, it is useful to assume that properties of liqui ...
... The glass transition A melt eventually transforms to a glass if crystallization is by-passed upon cooling. This transition from a liquid to a solid is accompanied by marked changes in second-order thermodynamic properties. To account for these changes, it is useful to assume that properties of liqui ...
Energy
... –a thermodynamic quantity equivalent to the total heat content of a system. –equal to the internal energy of the system plus the product of pressure and volume. •Enthalpy = H = E + PV ...
... –a thermodynamic quantity equivalent to the total heat content of a system. –equal to the internal energy of the system plus the product of pressure and volume. •Enthalpy = H = E + PV ...
On Free Energy and Internal Combustion Engine
... are as follows. Step 1. Reactant is inputted into the engine at ( P1 , T1 ) Net entropy change with respect to reactant at ( P1 , T1 ) = 0. Nothing has happened so far. Step 2. Reactant is compressed adiabatically and reversibly to ( PH , TH ) Net entropy change with respect to reactant at ( P1 , T1 ...
... are as follows. Step 1. Reactant is inputted into the engine at ( P1 , T1 ) Net entropy change with respect to reactant at ( P1 , T1 ) = 0. Nothing has happened so far. Step 2. Reactant is compressed adiabatically and reversibly to ( PH , TH ) Net entropy change with respect to reactant at ( P1 , T1 ...
Energetics Past Paper Questions
... State the name of the term ∆H˚. State, with a reason, whether reaction I would be accompanied by a decrease or increase in temperature. (3) At room temperature sulfur trioxide, SO3, is a solid. Deduce, with a reason, whether the ∆H˚ value would be more negative or less negative if SO3(s) instead of ...
... State the name of the term ∆H˚. State, with a reason, whether reaction I would be accompanied by a decrease or increase in temperature. (3) At room temperature sulfur trioxide, SO3, is a solid. Deduce, with a reason, whether the ∆H˚ value would be more negative or less negative if SO3(s) instead of ...
Document
... 1. In the reaction 2 H2(g) + O2(g) → 2 H2O(l), 3 mol of gas-phase molecules is replaced by 2 mol of liquid-phase molecules, so ∆ng = −3 mol. Therefore, at 298 K, when RT = 2.5 kJ mol−1, the enthalpy and internal energy changes taking place in the system are related by • Note that the difference is e ...
... 1. In the reaction 2 H2(g) + O2(g) → 2 H2O(l), 3 mol of gas-phase molecules is replaced by 2 mol of liquid-phase molecules, so ∆ng = −3 mol. Therefore, at 298 K, when RT = 2.5 kJ mol−1, the enthalpy and internal energy changes taking place in the system are related by • Note that the difference is e ...
Chm 118
... As chemists we most often want to express the value of a thermodynamic quantity for a reaction of some sort. Since the quantity of material that we deal with is variable, the most useful kind of quantity is an intensive one. What kind of property is entropy? What would happen if we expressed entropy ...
... As chemists we most often want to express the value of a thermodynamic quantity for a reaction of some sort. Since the quantity of material that we deal with is variable, the most useful kind of quantity is an intensive one. What kind of property is entropy? What would happen if we expressed entropy ...
Document
... • The – side of the H2O dipole is attracted to the Na+ ions while the + side of the H2O dipole heads for the Cl- ions. • The H2O molecules surround and carry off each ion, until the crystal is completely dissolved. ...
... • The – side of the H2O dipole is attracted to the Na+ ions while the + side of the H2O dipole heads for the Cl- ions. • The H2O molecules surround and carry off each ion, until the crystal is completely dissolved. ...
18.3 Standard Entropies and the Third Law of
... additional information is given for you to locate it. thermodynamics* study of the relationship between heat and other forms of energy involved in a chemical or physical process (chapter introduction) internal energy (U) ...
... additional information is given for you to locate it. thermodynamics* study of the relationship between heat and other forms of energy involved in a chemical or physical process (chapter introduction) internal energy (U) ...
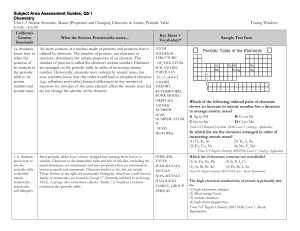
Subject Area Assessment Guides
... atoms can be almost entirely covalent, almost entirely ionic, or in between these two extremes. The triple bond in nitrogen molecules (N2) is nearly 100 percent covalent. A salt such as sodium chloride (NaCl) has bonds that are nearly completely ionic. However, the electrons in gaseous hydrogen chlo ...
... atoms can be almost entirely covalent, almost entirely ionic, or in between these two extremes. The triple bond in nitrogen molecules (N2) is nearly 100 percent covalent. A salt such as sodium chloride (NaCl) has bonds that are nearly completely ionic. However, the electrons in gaseous hydrogen chlo ...
Transition state theory
Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ‡Hɵ), the standard entropy of activation (Δ‡Sɵ), and the standard Gibbs energy of activation (Δ‡Gɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest at the transition state.)This theory was developed simultaneously in 1935 by Henry Eyring, then at Princeton University, and by Meredith Gwynne Evans and Michael Polanyi of the University of Manchester. TST is also referred to as ""activated-complex theory,"" ""absolute-rate theory,"" and ""theory of absolute reaction rates.""Before the development of TST, the Arrhenius rate law was widely used to determine energies for the reaction barrier. The Arrhenius equation derives from empirical observations and ignores any mechanistic considerations, such as whether one or more reactive intermediates are involved in the conversion of a reactant to a product. Therefore, further development was necessary to understand the two parameters associated with this law, the pre-exponential factor (A) and the activation energy (Ea). TST, which led to the Eyring equation, successfully addresses these two issues; however, 46 years elapsed between the publication of the Arrhenius rate law, in 1889, and the Eyring equation derived from TST, in 1935. During that period, many scientists and researchers contributed significantly to the development of the theory.