Ch 3 Chemical Reactions 2013-Sept-08
... A substance dissolved in water is an Aqueous Solution (aq) Law of Conservation of Matter = matter can neither be created or destroyed. Atoms are conserved in Chemical Reactions. The same elements and number of elements on the left side (the reactants) equals those on the right side (the products). I ...
... A substance dissolved in water is an Aqueous Solution (aq) Law of Conservation of Matter = matter can neither be created or destroyed. Atoms are conserved in Chemical Reactions. The same elements and number of elements on the left side (the reactants) equals those on the right side (the products). I ...
FoundationsofChemistryppt
... Explaining Chemical Reactions (cont.) • The formulas to the left of the arrow represent the reactants—the substances present before the reaction takes place. • The formulas to the right of the arrow represent the products—the new substances present after the reaction. • The arrow indicates that a r ...
... Explaining Chemical Reactions (cont.) • The formulas to the left of the arrow represent the reactants—the substances present before the reaction takes place. • The formulas to the right of the arrow represent the products—the new substances present after the reaction. • The arrow indicates that a r ...
35 - TAMU Chemistry
... Nitrogen Fixation: Name given to the reactions that microorganisms use to make NH3 from N2. Ammonia: • Sharp odor • Irritates lungs. Can cause death if inhaled in large quantities • Used as a fertilizer by injecting directly into the soil Haber – Bosch Process: catalysts ...
... Nitrogen Fixation: Name given to the reactions that microorganisms use to make NH3 from N2. Ammonia: • Sharp odor • Irritates lungs. Can cause death if inhaled in large quantities • Used as a fertilizer by injecting directly into the soil Haber – Bosch Process: catalysts ...
Fulltext PDF - Indian Academy of Sciences
... basis and then by single point PCM calculations at the same level but using the larger basis set. 2.3 Thermal energy and molecular entropy Thermal energy and entropy contribution towards the free energy change of the reductive process are obtained for the optimized geometry of a truncated model of t ...
... basis and then by single point PCM calculations at the same level but using the larger basis set. 2.3 Thermal energy and molecular entropy Thermal energy and entropy contribution towards the free energy change of the reductive process are obtained for the optimized geometry of a truncated model of t ...
Experimental and Computational Evidence of Metal‑O2 Activation
... upon exposure to the H2O2 product of enzyme turnover. Previous works have neglected this reaction and assumed that neither CoII nor CuII undergoes a change in redox state during enzyme catalysis.32 The Ered in eq 2 may involve complex internal redox chemistry in certain copper amine oxidases,33−36 w ...
... upon exposure to the H2O2 product of enzyme turnover. Previous works have neglected this reaction and assumed that neither CoII nor CuII undergoes a change in redox state during enzyme catalysis.32 The Ered in eq 2 may involve complex internal redox chemistry in certain copper amine oxidases,33−36 w ...
Advanced Placement Chemistry
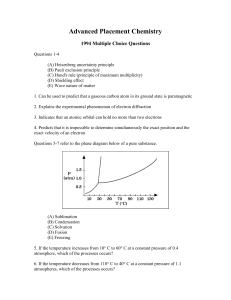
... (D) NH3 (E) BaCl2 66. What is the pH of a 1.0 x 10¯2-molar solution of HCN? (For HCN, Ka = 4.0 x 10¯10.) (A) 10 (B) Between 7 and 10 (C) 7 (D) Between 4 and 7 (E) 4 67. Substances X and Y that were in a solution were separated in the laboratory using the technique of fractional crystallization. This ...
... (D) NH3 (E) BaCl2 66. What is the pH of a 1.0 x 10¯2-molar solution of HCN? (For HCN, Ka = 4.0 x 10¯10.) (A) 10 (B) Between 7 and 10 (C) 7 (D) Between 4 and 7 (E) 4 67. Substances X and Y that were in a solution were separated in the laboratory using the technique of fractional crystallization. This ...
Transition state theory
Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ‡Hɵ), the standard entropy of activation (Δ‡Sɵ), and the standard Gibbs energy of activation (Δ‡Gɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest at the transition state.)This theory was developed simultaneously in 1935 by Henry Eyring, then at Princeton University, and by Meredith Gwynne Evans and Michael Polanyi of the University of Manchester. TST is also referred to as ""activated-complex theory,"" ""absolute-rate theory,"" and ""theory of absolute reaction rates.""Before the development of TST, the Arrhenius rate law was widely used to determine energies for the reaction barrier. The Arrhenius equation derives from empirical observations and ignores any mechanistic considerations, such as whether one or more reactive intermediates are involved in the conversion of a reactant to a product. Therefore, further development was necessary to understand the two parameters associated with this law, the pre-exponential factor (A) and the activation energy (Ea). TST, which led to the Eyring equation, successfully addresses these two issues; however, 46 years elapsed between the publication of the Arrhenius rate law, in 1889, and the Eyring equation derived from TST, in 1935. During that period, many scientists and researchers contributed significantly to the development of the theory.