Chemistry Standardized Test Practice: Student Edition
... Rutherford’s experiment in which he used a radioactive source to “shoot” alpha particles at a thin sheet of gold foil. Based on these results, what were Rutherford’s conclusions? ...
... Rutherford’s experiment in which he used a radioactive source to “shoot” alpha particles at a thin sheet of gold foil. Based on these results, what were Rutherford’s conclusions? ...
Thermodynamics: Entropy and Free Energy
... standard molar entropy, J/mol K. Table 19.1.1 shows standard molar entropy values for water at various temperatures. Interactive Figure 19.1.11 shows a generalized plot of the entropy of a substance at each temperature from 0 K to above its boiling point. Note in Table 19.1.1 and Interactive Figur ...
... standard molar entropy, J/mol K. Table 19.1.1 shows standard molar entropy values for water at various temperatures. Interactive Figure 19.1.11 shows a generalized plot of the entropy of a substance at each temperature from 0 K to above its boiling point. Note in Table 19.1.1 and Interactive Figur ...
Chemistry 12 Worksheet 2-3 Calculations Involving the
... PDFMAILER.COM Print and send PDF files as Emails with any application, ad-sponsored and free of charge www.pdfmailer.com ...
... PDFMAILER.COM Print and send PDF files as Emails with any application, ad-sponsored and free of charge www.pdfmailer.com ...
enthalpy change
... • We will be learning how to communicate enthalpy changes in four ways: 1. By stating the molar enthalpy of a specific reactant in a reaction 2. By stating the enthalpy change for a balanced reaction equation 3. By including an energy value as a term in a balanced reaction equation 4. By drawing a c ...
... • We will be learning how to communicate enthalpy changes in four ways: 1. By stating the molar enthalpy of a specific reactant in a reaction 2. By stating the enthalpy change for a balanced reaction equation 3. By including an energy value as a term in a balanced reaction equation 4. By drawing a c ...
Chapter 5
... Chemical reactions involve reactants undergoing chemical change to form new substances, products. reactants S products What is not apparent in the preceding equation is the role of energy in a reaction. For many reactions, energy, often in the form of heat, is absorbed—that is, it acts somewhat like ...
... Chemical reactions involve reactants undergoing chemical change to form new substances, products. reactants S products What is not apparent in the preceding equation is the role of energy in a reaction. For many reactions, energy, often in the form of heat, is absorbed—that is, it acts somewhat like ...
Stoichiometry of Chemical Reactions
... balancing a chemical equation. Consider as an example the reaction between one methane molecule (CH4) and two diatomic oxygen molecules (O2) to produce one carbon dioxide molecule (CO2) and two water molecules (H2O). The chemical equation representing this process is provided in the upper half of Fi ...
... balancing a chemical equation. Consider as an example the reaction between one methane molecule (CH4) and two diatomic oxygen molecules (O2) to produce one carbon dioxide molecule (CO2) and two water molecules (H2O). The chemical equation representing this process is provided in the upper half of Fi ...
(a) From , 2013 General Chemistry I
... (specify the conditions): (a) ΔU = 0 (b) Q = 0 (c) q < 0 (d) ΔU = q (e) ΔU = w Solution ...
... (specify the conditions): (a) ΔU = 0 (b) Q = 0 (c) q < 0 (d) ΔU = q (e) ΔU = w Solution ...
Chemical Reactivity of Ti+ within Water, Dimethyl Ether, and
... water vapor was combined with the Ar carrier gas, a reproducible TiO+ ion signal was observed during the repeated laser-vaporization pulses on the Ti target. This provides additional support for the suggestion that TiO+ forms through the reaction of Ti+ with water clusters. The observation in the ma ...
... water vapor was combined with the Ar carrier gas, a reproducible TiO+ ion signal was observed during the repeated laser-vaporization pulses on the Ti target. This provides additional support for the suggestion that TiO+ forms through the reaction of Ti+ with water clusters. The observation in the ma ...
The Magic of Matter
... Record the mass of your balloon and bottle together on the balance. (Be sure to take turns using the balance!) Put the funnel into your balloon. Scoop some sodium bicarbonate into the balloon. Remeasure the total mass of your balloon and soda bottle. Carefully stretch your balloon over the l ...
... Record the mass of your balloon and bottle together on the balance. (Be sure to take turns using the balance!) Put the funnel into your balloon. Scoop some sodium bicarbonate into the balloon. Remeasure the total mass of your balloon and soda bottle. Carefully stretch your balloon over the l ...
Reactions in Aqueous Solution (Brown 13th-Fossum
... CH3COOH (aq) + NaOH (aq) CH3COONa (aq) + H2O (l) When a strong acid reacts with a strong base, the net ionic equation is… HCl (aq) + NaOH (aq) NaCl (aq) + H2O (l) H+ (aq) + OH- (aq) H2O (l) Polyprotic acids (H2SO3,H2CO3, H3PO4) lose one H+ at a time; the first H+ is the easiest to lose. The ...
... CH3COOH (aq) + NaOH (aq) CH3COONa (aq) + H2O (l) When a strong acid reacts with a strong base, the net ionic equation is… HCl (aq) + NaOH (aq) NaCl (aq) + H2O (l) H+ (aq) + OH- (aq) H2O (l) Polyprotic acids (H2SO3,H2CO3, H3PO4) lose one H+ at a time; the first H+ is the easiest to lose. The ...
In Situ Soft X‑ray Absorption Spectroscopy Applied to Solid
... to π*CO orbital in the amide group has been reported in various amide compounds, e.g., formamide45 and malonamide.46 In Figure 2, a very small absorption peak corresponding to π*CO is also observed around 400 eV.15 All the absorption peaks observed in the C K-edge XAS (Figure 1) and N K-edge XAS ( ...
... to π*CO orbital in the amide group has been reported in various amide compounds, e.g., formamide45 and malonamide.46 In Figure 2, a very small absorption peak corresponding to π*CO is also observed around 400 eV.15 All the absorption peaks observed in the C K-edge XAS (Figure 1) and N K-edge XAS ( ...
Stoichiometric Problems III: Sto c o et c ob e s
... Here is a typical problem. problem If I have the following chemical reaction: N2(g) + 3H2 (g) = 2NH3 (g) And I start with 5 moles of N2 and 5 moles of H2, which chemical (H2 or N2) is the limiting reactant? My approach to solving this kind of problem is to calculate the amount of product that would ...
... Here is a typical problem. problem If I have the following chemical reaction: N2(g) + 3H2 (g) = 2NH3 (g) And I start with 5 moles of N2 and 5 moles of H2, which chemical (H2 or N2) is the limiting reactant? My approach to solving this kind of problem is to calculate the amount of product that would ...
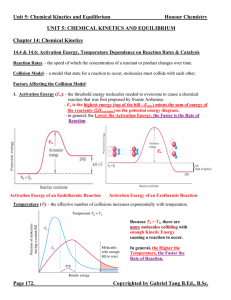
Unit 5: Chemical Kinetics and Equilibrium
... 1. When K >> 1, the equilibrium system favours the products. There are more products than reactants at the state of equilibrium. ([C]eq and [D]eq or PC, eq and PD, eq >> [A]eq and [B]eq or PA, eq and PB, eq) 2. When K << 1, the equilibrium system favours the reactants. There are less products than r ...
... 1. When K >> 1, the equilibrium system favours the products. There are more products than reactants at the state of equilibrium. ([C]eq and [D]eq or PC, eq and PD, eq >> [A]eq and [B]eq or PA, eq and PB, eq) 2. When K << 1, the equilibrium system favours the reactants. There are less products than r ...
Transition state theory
Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ‡Hɵ), the standard entropy of activation (Δ‡Sɵ), and the standard Gibbs energy of activation (Δ‡Gɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest at the transition state.)This theory was developed simultaneously in 1935 by Henry Eyring, then at Princeton University, and by Meredith Gwynne Evans and Michael Polanyi of the University of Manchester. TST is also referred to as ""activated-complex theory,"" ""absolute-rate theory,"" and ""theory of absolute reaction rates.""Before the development of TST, the Arrhenius rate law was widely used to determine energies for the reaction barrier. The Arrhenius equation derives from empirical observations and ignores any mechanistic considerations, such as whether one or more reactive intermediates are involved in the conversion of a reactant to a product. Therefore, further development was necessary to understand the two parameters associated with this law, the pre-exponential factor (A) and the activation energy (Ea). TST, which led to the Eyring equation, successfully addresses these two issues; however, 46 years elapsed between the publication of the Arrhenius rate law, in 1889, and the Eyring equation derived from TST, in 1935. During that period, many scientists and researchers contributed significantly to the development of the theory.