spontaneous change: entropy and free energy
... where S is the entropy, k is the Boltzmann constant, and W is the number of microstates. We can think of the Boltzmann constant as the gas constant per molecule; that is, k = R>NA . (Although we didn’t specifically introduce k in the discussion of kinetic–molecular theory, R>NA appears in equation 6 ...
... where S is the entropy, k is the Boltzmann constant, and W is the number of microstates. We can think of the Boltzmann constant as the gas constant per molecule; that is, k = R>NA . (Although we didn’t specifically introduce k in the discussion of kinetic–molecular theory, R>NA appears in equation 6 ...
The Theory of Intermolecular Forces
... using. This simplified picture is then like a landscape, with hills and valleys. There are energy minima on the potential energy surface that correspond to depressions in the landscape. In the real landscape such depressions are usually filled with water, to give lakes or tarns; there may be many of t ...
... using. This simplified picture is then like a landscape, with hills and valleys. There are energy minima on the potential energy surface that correspond to depressions in the landscape. In the real landscape such depressions are usually filled with water, to give lakes or tarns; there may be many of t ...
An experimentally validated numerical model of interface advance of
... Received: 18 June 2015 / Accepted: 12 December 2015 / Published online: 5 January 2016 Ó The Author(s) 2015. This article is published with open access at Springerlink.com ...
... Received: 18 June 2015 / Accepted: 12 December 2015 / Published online: 5 January 2016 Ó The Author(s) 2015. This article is published with open access at Springerlink.com ...
Section 6.3 Balancing Chemical Equations
... • an uncombined element replaces an element that is part of a compound • whether one metal will displace another metal from a compound can be determined by the relative reactivities of the 2 metals. The activity series of metals lists metals in order of decreasing reactivity. A reactive metal will r ...
... • an uncombined element replaces an element that is part of a compound • whether one metal will displace another metal from a compound can be determined by the relative reactivities of the 2 metals. The activity series of metals lists metals in order of decreasing reactivity. A reactive metal will r ...
Mole Ratio and Mass IP
... 5. Convert moles to grams (again, using atomic or molar masses) Example: Propane, C3H8, when used as a fuel, reacts with oxygen gas to produce carbon dioxide and water. What mass of oxygen gas will be required to react exactly with 96.1g of propane? ...
... 5. Convert moles to grams (again, using atomic or molar masses) Example: Propane, C3H8, when used as a fuel, reacts with oxygen gas to produce carbon dioxide and water. What mass of oxygen gas will be required to react exactly with 96.1g of propane? ...
chemistry
... Directions (66–83): Record your answers in the spaces provided in your answer booklet. Some questions may require the use of the Reference Tables for Physical Setting/Chemistry. Base your answers to questions 66 through 68 on the information below. In the early 1800s, John Dalton proposed an atomic ...
... Directions (66–83): Record your answers in the spaces provided in your answer booklet. Some questions may require the use of the Reference Tables for Physical Setting/Chemistry. Base your answers to questions 66 through 68 on the information below. In the early 1800s, John Dalton proposed an atomic ...
What are Physical Properties and Changes? - Mamanakis
... Chemical properties are any of the properties of matter that may only be observed and measured by performing a chemical change or chemical reaction. Chemical properties cannot be determined by touching or viewing a sample; the structure of the sample must be altered for the chemical properties to be ...
... Chemical properties are any of the properties of matter that may only be observed and measured by performing a chemical change or chemical reaction. Chemical properties cannot be determined by touching or viewing a sample; the structure of the sample must be altered for the chemical properties to be ...
U3 Student Workbook - The Connected Chemistry Curriculum
... of Mass to chemical equations by learning how to balance them. Following a teacher demonstration of the simulation and procedures, students will use the simulations to look at ten different reactions. In each of the reactions, students will create submicroscopic sketches and balance the chemical for ...
... of Mass to chemical equations by learning how to balance them. Following a teacher demonstration of the simulation and procedures, students will use the simulations to look at ten different reactions. In each of the reactions, students will create submicroscopic sketches and balance the chemical for ...
organic chemistry i - cm2113
... Tests or work missed due to unexcused but justifiable absences may be made up but for less than full credit. These usually are planned absences and the instructor is informed beforehand. The instructor will have to decide how a missed test or work is to be made up. Tardiness is a form of absenteeism ...
... Tests or work missed due to unexcused but justifiable absences may be made up but for less than full credit. These usually are planned absences and the instructor is informed beforehand. The instructor will have to decide how a missed test or work is to be made up. Tardiness is a form of absenteeism ...
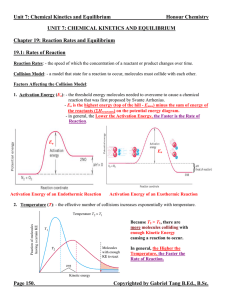
Unit 7 Reaction Rates and Equilibrium Notes
... Le Châtelier’s Principle: - a qualitative method to predict the shift on an equilibrium system if it is disturbed by means of changing concentration, pressure and temperature. - the equilibrium will shift in the direction that minimizes the change imposed on the system. 1. Effects of a Change in Con ...
... Le Châtelier’s Principle: - a qualitative method to predict the shift on an equilibrium system if it is disturbed by means of changing concentration, pressure and temperature. - the equilibrium will shift in the direction that minimizes the change imposed on the system. 1. Effects of a Change in Con ...
Answers to Selected Questions and Problems
... Anna and Bill would have observed kinetic energy from the movement of the welder and the motion of the sparks. The sparks would have glowed, indicating heat, light, and chemical energy. The molecules in image A have greater kinetic energy because they are moving faster. Any object that would move if ...
... Anna and Bill would have observed kinetic energy from the movement of the welder and the motion of the sparks. The sparks would have glowed, indicating heat, light, and chemical energy. The molecules in image A have greater kinetic energy because they are moving faster. Any object that would move if ...
Transition state theory
Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ‡Hɵ), the standard entropy of activation (Δ‡Sɵ), and the standard Gibbs energy of activation (Δ‡Gɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest at the transition state.)This theory was developed simultaneously in 1935 by Henry Eyring, then at Princeton University, and by Meredith Gwynne Evans and Michael Polanyi of the University of Manchester. TST is also referred to as ""activated-complex theory,"" ""absolute-rate theory,"" and ""theory of absolute reaction rates.""Before the development of TST, the Arrhenius rate law was widely used to determine energies for the reaction barrier. The Arrhenius equation derives from empirical observations and ignores any mechanistic considerations, such as whether one or more reactive intermediates are involved in the conversion of a reactant to a product. Therefore, further development was necessary to understand the two parameters associated with this law, the pre-exponential factor (A) and the activation energy (Ea). TST, which led to the Eyring equation, successfully addresses these two issues; however, 46 years elapsed between the publication of the Arrhenius rate law, in 1889, and the Eyring equation derived from TST, in 1935. During that period, many scientists and researchers contributed significantly to the development of the theory.