n - Physics
... it glitters as the diamond is moved about. Why does a diamond exhibit such brilliance? Why does it lose much of its brilliance when placed under water? ...
... it glitters as the diamond is moved about. Why does a diamond exhibit such brilliance? Why does it lose much of its brilliance when placed under water? ...
One Dimensionally Periodic Dielectric Reflectors from Self Assembled Block Copolymer-Homopolymer Blends
... ultrathin sections from the films using a Reichert-Jung cryoultramicrotome and stained them with OsO4 to provide mass thickness contrast of the microdomain structure. Transmission electron micrographs were obtained with JEOL 2000FX. Bright field images were used to determine the spatial characterist ...
... ultrathin sections from the films using a Reichert-Jung cryoultramicrotome and stained them with OsO4 to provide mass thickness contrast of the microdomain structure. Transmission electron micrographs were obtained with JEOL 2000FX. Bright field images were used to determine the spatial characterist ...
Light - PhysicsArts
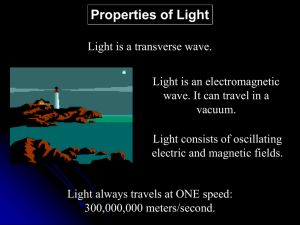
... Glowing with visible light from high temperatures Examples: flames, incandescent light bulbs Produce light via electromagnetic waves Electrical charge is accelerated by external force Acceleration produces wave consisting of ...
... Glowing with visible light from high temperatures Examples: flames, incandescent light bulbs Produce light via electromagnetic waves Electrical charge is accelerated by external force Acceleration produces wave consisting of ...
solution - UC Davis Physics
... wavelength change? iii) as approrpriate does either the frequency or wavelength get bigger or smaller? i) ii) iii) ...
... wavelength change? iii) as approrpriate does either the frequency or wavelength get bigger or smaller? i) ii) iii) ...
Meade® Ultra-High Transmission Coatings (UHTCTM) Group
... Observations of the Moon and planets, since they are observed in reflected (white) sunlight, benefit in image brightness from the full spectrum of increased transmission. The overall effect of the UHTC is, as it relates to image brightness, to increase the telescope’s effective aperture. Image brigh ...
... Observations of the Moon and planets, since they are observed in reflected (white) sunlight, benefit in image brightness from the full spectrum of increased transmission. The overall effect of the UHTC is, as it relates to image brightness, to increase the telescope’s effective aperture. Image brigh ...
intro to spectroscopy - Mount Holyoke College
... "signatures" of the atoms in that star when viewed through a spectroscope. Other common light emitting objects include jellyfish (which contain a protein, "green fluorescent protein" or GFP, which emits light when activated by the jellyfish's nervous system) and glowing safety light sticks (in which ...
... "signatures" of the atoms in that star when viewed through a spectroscope. Other common light emitting objects include jellyfish (which contain a protein, "green fluorescent protein" or GFP, which emits light when activated by the jellyfish's nervous system) and glowing safety light sticks (in which ...
Diffraction Links
... • This soap film varies in thickness and produces a rainbow of colors. • The top part is so thin it looks black. • All colors destructively interfere there. ...
... • This soap film varies in thickness and produces a rainbow of colors. • The top part is so thin it looks black. • All colors destructively interfere there. ...
Meade® Ultra-High Transmission Coatings (UHTCTM) Group
... dioxide (SiO2). The thickness of each coating layer precisely controlled to within ±1% of optimal thickness. The result is a dramatic increase in mirror reflectivity across the entire visible spectrum; at the important hydrogen-alpha wavelength of 656nm. — the predominant wavelength of emission nebu ...
... dioxide (SiO2). The thickness of each coating layer precisely controlled to within ±1% of optimal thickness. The result is a dramatic increase in mirror reflectivity across the entire visible spectrum; at the important hydrogen-alpha wavelength of 656nm. — the predominant wavelength of emission nebu ...
Exploring Sound and Light
... Pulsating lights are common in our world, but often go unnoticed. We are generally not aware that common light sources such as neon and fluorescent lamps flash on and off. You can observe this flashing by attaching an inexpensive neon night light to the end of an extension cord. In a dark room, care ...
... Pulsating lights are common in our world, but often go unnoticed. We are generally not aware that common light sources such as neon and fluorescent lamps flash on and off. You can observe this flashing by attaching an inexpensive neon night light to the end of an extension cord. In a dark room, care ...
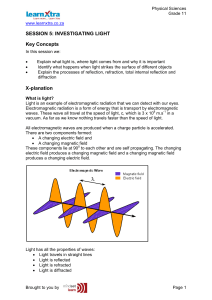
Electromagnetic Spectrum
... the light year to measure distances in space. A light year is the distance light travels in a year. • Astronomers have learned that all known stars, including the sun, have a nearly identical chemical composition. An astronomer analyzing a star or another component of the universe often starts by st ...
... the light year to measure distances in space. A light year is the distance light travels in a year. • Astronomers have learned that all known stars, including the sun, have a nearly identical chemical composition. An astronomer analyzing a star or another component of the universe often starts by st ...
Student Worksheet The Chemistry of Water Quality Tests
... After viewing the video, students will be asked to extend the ideas presented in the video and their research, presenting the data gathered from the websites to provide evidence for the following: a. The design and/or interpretation of the results of a separation experiment (filtration, paper chroma ...
... After viewing the video, students will be asked to extend the ideas presented in the video and their research, presenting the data gathered from the websites to provide evidence for the following: a. The design and/or interpretation of the results of a separation experiment (filtration, paper chroma ...
Matter
... composition, its structure, its properties, and the changes it undergoes (via reactions). Matter: anything that occupies space and has mass Mass pertains to the quantity of matter that an object has unaffected by location defined as resistance to acceleration ...
... composition, its structure, its properties, and the changes it undergoes (via reactions). Matter: anything that occupies space and has mass Mass pertains to the quantity of matter that an object has unaffected by location defined as resistance to acceleration ...
Photopolymer
A photopolymer is a polymer that changes its properties when exposed to light, often in the ultraviolet or visible region of the electromagnetic spectrum. These changes are often manifested structurally, for example hardening of the material occurs as a result of cross-linking when exposed to light. An example is shown below depicting a mixture of monomers, oligomers, and photoinitiators that conform into a hardened polymeric material through a process called curing,.A wide variety of technologically useful applications rely on photopolymers, for example some enamels and varnishes depend on photopolymer formulation for proper hardening upon exposure to light. In some instances, an enamel can cure in a fraction of a second when exposed to light, as opposed to thermally cured enamels which can require half an hour or longer. Curable materials are widely used for medical, printing, and photoresist technologies. Changes in structural and chemical properties can be induced internally by chromophores that the polymer subunit already possesses, or externally by addition of photosensitive molecules. Typically a photopolymer consists of a mixture of multifunctional monomers and oligomers in order to achieve the desired physical properties, and therefore a wide variety of monomers and oligomers have been developed that can polymerize in the presence of light either through internal or external initiation. Photopolymers undergo a process called curing, where oligomers are cross-linked upon exposure to light, forming what is known as a network polymer. The result of photo curing is the formation of a thermoset network of polymers. One of the advantages of photo-curing is that it can be done selectively using high energy light sources, for example lasers, however, most systems are not readily activated by light, and in this case a photoinitiator is required. Photoinitiators are compounds that upon radiation of light decompose into reactive species that activate polymerization of specific functional groups on the oligomers. An example of a mixture that undergoes cross-linking when exposed to light is shown below. The mixture consists of monomeric styrene and oligomeric acrylates.Most commonly, photopolymerized systems are typically cured through UV radiation, since ultraviolet light is more energetic; however, the development of dye-based photoinitiator systems have allowed for the use of visible light, having potential advantages of processes that are more simple and safe to handle. UV curing in industrial processes has greatly expanded over the past several decades. Many traditional thermally cured and solvent-based technologies can be replaced by photopolymerization technologies. The advantages of photopolymerization over thermally cured polymerization include high rates of polymerization and environmental benefits from elimination of volatile organic solvents.There are two general routes for photoinitiation: free radical and ionic. The general process involves doping a batch of neat polymer with small amounts of photoinitiator, followed by selective radiation of light, resulting a highly cross-linked product. Many of these reactions do not require solvent which eliminates termination path via reaction of initiators with solvent and impurities, in addition to decreasing the overall cost.