Eells Reveals the Mystery of the Healing Light
... action occurring. But we believe that in all the medical applications benefiting from near IR light, it is the interaction of the cytochrome oxidase with the light that is the cause of the improvement.” Since first publishing on NIR therapy on methanol-induced blindness, Eells and Whelan also have w ...
... action occurring. But we believe that in all the medical applications benefiting from near IR light, it is the interaction of the cytochrome oxidase with the light that is the cause of the improvement.” Since first publishing on NIR therapy on methanol-induced blindness, Eells and Whelan also have w ...
Slides - PDF - University of Toronto Physics
... which causes red light to be a little more strongly absorbed in water than blue light. • Red light is reduced to one-quarter of its initial brightness by 15 meters of water. There is very little red light in the sunlight that penetrates below 30 meters of water. • When red is removed from white ligh ...
... which causes red light to be a little more strongly absorbed in water than blue light. • Red light is reduced to one-quarter of its initial brightness by 15 meters of water. There is very little red light in the sunlight that penetrates below 30 meters of water. • When red is removed from white ligh ...
Transmission Measurements of Polymer Thin Films
... feasibility measurement with a standard system can easily set you on the right path to choosing the optimal system configuration. For those who need more from an interference measurement, reflectometry may be the best solution. A system such as the NanoCalc Thin Film Reflectometer takes the next ste ...
... feasibility measurement with a standard system can easily set you on the right path to choosing the optimal system configuration. For those who need more from an interference measurement, reflectometry may be the best solution. A system such as the NanoCalc Thin Film Reflectometer takes the next ste ...
Chapter 1 Introduction to Chemistry
... wind eroding rocks – PHYSICAL CHANGE dead leaves decaying – CHEMICAL CHANGE rain puddle drying up – PHYSICAL CHANGE mixing flour and baking powder- PHYSICAL CHANGE gasoline evaporating – PHYSICAL CHANGE hydrogen peroxide decomposing- CHEMICAL CHANGE bread baking in an oven – CHEMICAL CHANGE instant ...
... wind eroding rocks – PHYSICAL CHANGE dead leaves decaying – CHEMICAL CHANGE rain puddle drying up – PHYSICAL CHANGE mixing flour and baking powder- PHYSICAL CHANGE gasoline evaporating – PHYSICAL CHANGE hydrogen peroxide decomposing- CHEMICAL CHANGE bread baking in an oven – CHEMICAL CHANGE instant ...
IR Workshop Poster - Beamline Presentation
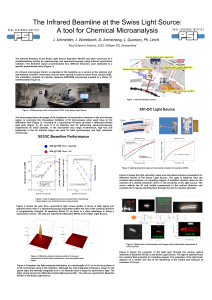
... The Infrared beamline at the Swiss Light Source (beamline X01DC) has been conceived as a multidisciplinary facility for spectroscopy and spectromicroscopy using infrared synchrotron radiation. The beamline layout accommodates four different branches, each dedicated to a specific experimental setup ( ...
... The Infrared beamline at the Swiss Light Source (beamline X01DC) has been conceived as a multidisciplinary facility for spectroscopy and spectromicroscopy using infrared synchrotron radiation. The beamline layout accommodates four different branches, each dedicated to a specific experimental setup ( ...
Chapter 37 : Interference of Light
... You Try Intensity In a double-slit experiment, the distance between the slits is 0.2 mm, and the distance to the screen is 150 cm. What wavelength (in nm) is needed to have the intensity at a point 1 mm from the central maximum on the screen be 80% of the maximum intensity? a. 900 ...
... You Try Intensity In a double-slit experiment, the distance between the slits is 0.2 mm, and the distance to the screen is 150 cm. What wavelength (in nm) is needed to have the intensity at a point 1 mm from the central maximum on the screen be 80% of the maximum intensity? a. 900 ...
Lab#8RayOpticsNew
... light have different speeds in transparent objects. The following steps will help you determine what color of light moves fastest, slowest, and how this determines what rainbows look like. The index of refraction is related to the refraction angle of the incident light beam. The greater the angle of ...
... light have different speeds in transparent objects. The following steps will help you determine what color of light moves fastest, slowest, and how this determines what rainbows look like. The index of refraction is related to the refraction angle of the incident light beam. The greater the angle of ...
Lecture 36 Newton on Ether
... soon as the Thermometer which is not in vacuo. And when the Vessels are carried back into a cold place, the Thermometer in vacuo will grow cold almost as soon as the other Thermometer. ...
... soon as the Thermometer which is not in vacuo. And when the Vessels are carried back into a cold place, the Thermometer in vacuo will grow cold almost as soon as the other Thermometer. ...
Document
... Refraction At the surface of transparent media, glass, water etc both reflection and refraction occur. Refraction (deflection from a straight path in passing obliquely from one medium ( such as air) into another (such as glass) Incident Ray Medium 1 Medium 2 ...
... Refraction At the surface of transparent media, glass, water etc both reflection and refraction occur. Refraction (deflection from a straight path in passing obliquely from one medium ( such as air) into another (such as glass) Incident Ray Medium 1 Medium 2 ...
Refraction - Snell`s Law, Internal Reflection, Dispersion (PowerPoint)
... A typical fiber thickness might be in the range of say, 50m, just about the thickness of a human head-hair. An optical fiber is like a light pipe. Light entering it at the proper angle will zig zag its way through it as many as 15000 times paer meter without being lost through the walls of the fibe ...
... A typical fiber thickness might be in the range of say, 50m, just about the thickness of a human head-hair. An optical fiber is like a light pipe. Light entering it at the proper angle will zig zag its way through it as many as 15000 times paer meter without being lost through the walls of the fibe ...
20170515_final_higher_revision
... The frequency of a wave is determined by the source that generated the wave. As a consequence of this, no matter what happens to a wave after it has been generated, its frequency does not change. This means that, when a light wave passes from one medium into another (when it is refracted) its freque ...
... The frequency of a wave is determined by the source that generated the wave. As a consequence of this, no matter what happens to a wave after it has been generated, its frequency does not change. This means that, when a light wave passes from one medium into another (when it is refracted) its freque ...
Chemistry Lesson 10 Describing Matter
... 2. Mass, volume, length, etc are examples of extensive properties. 3. These are things which can be measured physically, and the measurement would change if there was more or less of the matter present 4. For example, 10 cm3 of water weighs 10 grams. If I take away some water, it weighs less. ...
... 2. Mass, volume, length, etc are examples of extensive properties. 3. These are things which can be measured physically, and the measurement would change if there was more or less of the matter present 4. For example, 10 cm3 of water weighs 10 grams. If I take away some water, it weighs less. ...
Matter – Properties and Changes 1 Intensive properties
... Ability of a substance to combine with or change into 1 or more substances AKA: chemical reaction – o new substance formed in the reaction that has different composition and properties from the original o Crushing grapes physical change Fermenting grape juice and sugars into wine chemical change ...
... Ability of a substance to combine with or change into 1 or more substances AKA: chemical reaction – o new substance formed in the reaction that has different composition and properties from the original o Crushing grapes physical change Fermenting grape juice and sugars into wine chemical change ...
Chapter 23 – Wave Optics
... In the center of the screen the light coming from the two slits travels the same distance. They are thus in phase and add constructively, giving a bright line. Above and below the center line the light arriving from the two slits travel different distances and will be either in phase or out of ph ...
... In the center of the screen the light coming from the two slits travels the same distance. They are thus in phase and add constructively, giving a bright line. Above and below the center line the light arriving from the two slits travel different distances and will be either in phase or out of ph ...
Unit 2 Review: Chemistry - Mr. Hoover's Science Classes
... atoms and how they combine to form all types of matter. Atomic theory helps us to understand why there are different kinds of atoms. It explains how atoms combine to form over 100 known elements and all other forms of matter, including compounds and ...
... atoms and how they combine to form all types of matter. Atomic theory helps us to understand why there are different kinds of atoms. It explains how atoms combine to form over 100 known elements and all other forms of matter, including compounds and ...
Light Study Guide
... Why Do Objects Appear to be a Certain Color? When objects do not absorb a wavelength of light, they reflect it. The color that is reflected is the color an object appears to be. For example, white light shines on a green leaf. The leaf absorbs the entire visible spectrum of light energy except for l ...
... Why Do Objects Appear to be a Certain Color? When objects do not absorb a wavelength of light, they reflect it. The color that is reflected is the color an object appears to be. For example, white light shines on a green leaf. The leaf absorbs the entire visible spectrum of light energy except for l ...
CHAPTER - 11 THE HUMAN EYE AND THE COLOURFUL WORLD
... When a beam of white light is passed through a glass prism, it is split up into a band of colours called spectrum. This is called dispersion of white light. The spectrum of white has the colours violet, indigo, blue, green, yellow, orange and red (VIBGYOR). The red light bends the least and the viol ...
... When a beam of white light is passed through a glass prism, it is split up into a band of colours called spectrum. This is called dispersion of white light. The spectrum of white has the colours violet, indigo, blue, green, yellow, orange and red (VIBGYOR). The red light bends the least and the viol ...
Slide 1
... When a beam of white light is passed through a glass prism, it is split up into a band of colours called spectrum. This is called dispersion of white light. The spectrum of white has the colours violet, indigo, blue, green, yellow, orange and red (VIBGYOR). The red light bends the least and the viol ...
... When a beam of white light is passed through a glass prism, it is split up into a band of colours called spectrum. This is called dispersion of white light. The spectrum of white has the colours violet, indigo, blue, green, yellow, orange and red (VIBGYOR). The red light bends the least and the viol ...
2-21 The Nature of Electromagnetic Waves
... How the electrons in matter interact with light, is largely determined by the degree to which the electrons are bound in the matter. As a rather bizarre example of how a large number of complicated interactions can combine to form a simple total effect, the mix of attractive and repulsive 1/r 2 Coul ...
... How the electrons in matter interact with light, is largely determined by the degree to which the electrons are bound in the matter. As a rather bizarre example of how a large number of complicated interactions can combine to form a simple total effect, the mix of attractive and repulsive 1/r 2 Coul ...
Photopolymer
A photopolymer is a polymer that changes its properties when exposed to light, often in the ultraviolet or visible region of the electromagnetic spectrum. These changes are often manifested structurally, for example hardening of the material occurs as a result of cross-linking when exposed to light. An example is shown below depicting a mixture of monomers, oligomers, and photoinitiators that conform into a hardened polymeric material through a process called curing,.A wide variety of technologically useful applications rely on photopolymers, for example some enamels and varnishes depend on photopolymer formulation for proper hardening upon exposure to light. In some instances, an enamel can cure in a fraction of a second when exposed to light, as opposed to thermally cured enamels which can require half an hour or longer. Curable materials are widely used for medical, printing, and photoresist technologies. Changes in structural and chemical properties can be induced internally by chromophores that the polymer subunit already possesses, or externally by addition of photosensitive molecules. Typically a photopolymer consists of a mixture of multifunctional monomers and oligomers in order to achieve the desired physical properties, and therefore a wide variety of monomers and oligomers have been developed that can polymerize in the presence of light either through internal or external initiation. Photopolymers undergo a process called curing, where oligomers are cross-linked upon exposure to light, forming what is known as a network polymer. The result of photo curing is the formation of a thermoset network of polymers. One of the advantages of photo-curing is that it can be done selectively using high energy light sources, for example lasers, however, most systems are not readily activated by light, and in this case a photoinitiator is required. Photoinitiators are compounds that upon radiation of light decompose into reactive species that activate polymerization of specific functional groups on the oligomers. An example of a mixture that undergoes cross-linking when exposed to light is shown below. The mixture consists of monomeric styrene and oligomeric acrylates.Most commonly, photopolymerized systems are typically cured through UV radiation, since ultraviolet light is more energetic; however, the development of dye-based photoinitiator systems have allowed for the use of visible light, having potential advantages of processes that are more simple and safe to handle. UV curing in industrial processes has greatly expanded over the past several decades. Many traditional thermally cured and solvent-based technologies can be replaced by photopolymerization technologies. The advantages of photopolymerization over thermally cured polymerization include high rates of polymerization and environmental benefits from elimination of volatile organic solvents.There are two general routes for photoinitiation: free radical and ionic. The general process involves doping a batch of neat polymer with small amounts of photoinitiator, followed by selective radiation of light, resulting a highly cross-linked product. Many of these reactions do not require solvent which eliminates termination path via reaction of initiators with solvent and impurities, in addition to decreasing the overall cost.