year group: 5 - Priory Fields School, Dover
... Sketch a cartoon on the board showing a child on one side of the outside corner of a building shining a torch at night time. Around the other side of the corner draw his friend. In a speech bubble write; ‘The child cannot see the other one child because light from the torch cannot pass through the d ...
... Sketch a cartoon on the board showing a child on one side of the outside corner of a building shining a torch at night time. Around the other side of the corner draw his friend. In a speech bubble write; ‘The child cannot see the other one child because light from the torch cannot pass through the d ...
MT 1 Answers Version C - The University of Oklahoma Department
... 30. How does the speed of light traveling through a medium (such as air or glass) compare to the speed of light in a vacuum? (a) ...
... 30. How does the speed of light traveling through a medium (such as air or glass) compare to the speed of light in a vacuum? (a) ...
MT 1 Answers Version B
... Choose the answer that best completes the question. Read each problem carefully and read through all the answers. Take your time. If a question is unclear, ask for clarification during the exam. Mark your answers on the scantron sheet and on your copy of the exam. Keep your copy of the exam and chec ...
... Choose the answer that best completes the question. Read each problem carefully and read through all the answers. Take your time. If a question is unclear, ask for clarification during the exam. Mark your answers on the scantron sheet and on your copy of the exam. Keep your copy of the exam and chec ...
Matter and Energy
... • Extensive properties are dependent upon the amount of substance present. Ex- mass, length • Intensive property is independent of the amount of substance present. Ex- density, temperature ...
... • Extensive properties are dependent upon the amount of substance present. Ex- mass, length • Intensive property is independent of the amount of substance present. Ex- density, temperature ...
Chapter 11 Self Quiz Answers
... (c)(ii) Fireflies and certain other organisms can produce light as a result of a chemical reaction that takes place inside their bodies, or bioluminescence; (d)(v) In an energy-efficient fluorescent light bulb, a ...
... (c)(ii) Fireflies and certain other organisms can produce light as a result of a chemical reaction that takes place inside their bodies, or bioluminescence; (d)(v) In an energy-efficient fluorescent light bulb, a ...
Characteristic Properties Non-Characteristic Properties
... • These can be observed or measured without changing the make-up of the matter of the object • These properties can be used to describe the object • Two categories: non-characteristic and characteristic properties ...
... • These can be observed or measured without changing the make-up of the matter of the object • These properties can be used to describe the object • Two categories: non-characteristic and characteristic properties ...
law of reflection - Science with Ms. Tantri
... • All sight lines (viewers) will intersect at the image location • The image is therefore formed at the single point on the opposite side of the mirror from where all the light rays appear to have diverged Viewer 1 ...
... • All sight lines (viewers) will intersect at the image location • The image is therefore formed at the single point on the opposite side of the mirror from where all the light rays appear to have diverged Viewer 1 ...
Solar exposure condition improvement in urban area using light guide
... remote from the sun light without taking the space by heavy-section light guides. One of the disadvantages of using these light guides is impossibility to work without active heliostats. In terms of operating of these plants, it is additional costs for energy as well as for the routine maintenance o ...
... remote from the sun light without taking the space by heavy-section light guides. One of the disadvantages of using these light guides is impossibility to work without active heliostats. In terms of operating of these plants, it is additional costs for energy as well as for the routine maintenance o ...
index of refraction
... Where n1 is the index of refraction for the medium of the incident light, a is the incident angle, n2 is the index of refraction of the material that the light is entering, and b is the angle of refraction. According to this law, if the angle of incidence is changed then the angle of refraction must ...
... Where n1 is the index of refraction for the medium of the incident light, a is the incident angle, n2 is the index of refraction of the material that the light is entering, and b is the angle of refraction. According to this law, if the angle of incidence is changed then the angle of refraction must ...
November 11th Electromagnetic Waves - Chapter 34
... materials rotate the plane of polarization ! The rotation angle may depends on the frequency (color) ! This is due to molecular asymmetry e.g. molecules with spiral shapes ! Karo syrup and scotch tape ...
... materials rotate the plane of polarization ! The rotation angle may depends on the frequency (color) ! This is due to molecular asymmetry e.g. molecules with spiral shapes ! Karo syrup and scotch tape ...
- Adlershof.de
... life. Adlershof, Germany’s largest science and technology park, ranks among the world’s leading sites for research and development of light-based technologies. It is currently home to six research institutes and 70 companies that are active in photonics, optics, and photovoltaics. They cover almost ...
... life. Adlershof, Germany’s largest science and technology park, ranks among the world’s leading sites for research and development of light-based technologies. It is currently home to six research institutes and 70 companies that are active in photonics, optics, and photovoltaics. They cover almost ...
compound - Coal City Unit #1
... • molecular formula - C12H22O11 (sugar) • ionic formula – NaCl (table salt) • shows numbers of atoms of ea. elem. present in ...
... • molecular formula - C12H22O11 (sugar) • ionic formula – NaCl (table salt) • shows numbers of atoms of ea. elem. present in ...
Document
... • A prism shows visible light spread out into colors (ROY G. BIV) • Herschel showed (c. 1800) that there is energy outside the visible spectrum, by placing a thermometer beyond the red (infrared radiation) • EM radiation exists at all possible wavelengths and frequencies • Visible radiation runs fro ...
... • A prism shows visible light spread out into colors (ROY G. BIV) • Herschel showed (c. 1800) that there is energy outside the visible spectrum, by placing a thermometer beyond the red (infrared radiation) • EM radiation exists at all possible wavelengths and frequencies • Visible radiation runs fro ...
Introduction to Chemistry and Measurement
... close together but not in fixed positions Gas – neither definite volume nor definite shape; particles are at great distances from one another Plasma – high temperature, ionized phase of matter as found on the sun. ...
... close together but not in fixed positions Gas – neither definite volume nor definite shape; particles are at great distances from one another Plasma – high temperature, ionized phase of matter as found on the sun. ...
Chapter 1 Reading Guide
... Guidelines for determining the number of significant figures in a measured quantity are: • The number of significant figures is the number of digits known with certainty plus one uncertain digit. (Example: 2.2405 g means we are sure the mass is 2.240 g but we are uncertain about the nearest 0.0001 g ...
... Guidelines for determining the number of significant figures in a measured quantity are: • The number of significant figures is the number of digits known with certainty plus one uncertain digit. (Example: 2.2405 g means we are sure the mass is 2.240 g but we are uncertain about the nearest 0.0001 g ...
The Nature of Light
... The Electromagnetic Spectrum, continued • Infrared light can be felt as warmth. – Infrared (IR) wavelengths are slightly longer than red visible light. • Sunlight contains ultraviolet light. – The invisible light that lies just beyond violet light falls into the ultraviolet (UV) portion of the spect ...
... The Electromagnetic Spectrum, continued • Infrared light can be felt as warmth. – Infrared (IR) wavelengths are slightly longer than red visible light. • Sunlight contains ultraviolet light. – The invisible light that lies just beyond violet light falls into the ultraviolet (UV) portion of the spect ...
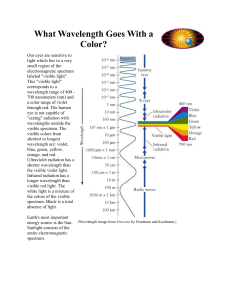
What Wavelength Goes With a Color?
... Energy with wavelengths too short to see is "bluer than blue". Light with such short wavelengths is called "Ultraviolet" light. How do we know this light exists? One way is that this kind of light causes sunburns. Our skin is sensitive to this kind of light. If we stay out in this light without sunb ...
... Energy with wavelengths too short to see is "bluer than blue". Light with such short wavelengths is called "Ultraviolet" light. How do we know this light exists? One way is that this kind of light causes sunburns. Our skin is sensitive to this kind of light. If we stay out in this light without sunb ...
Chapter 21
... • Optical properties of non-Metals: -- for Egap < 1.8 eV, absorption of all wavelengths of light radiation -- for Egap > 3.1 eV, no absorption of visible light radiation -- for 1.8 eV < Egap < 3.1 eV, absorption of some range of light radiation wavelengths -- color determined by wavelength distribut ...
... • Optical properties of non-Metals: -- for Egap < 1.8 eV, absorption of all wavelengths of light radiation -- for Egap > 3.1 eV, no absorption of visible light radiation -- for 1.8 eV < Egap < 3.1 eV, absorption of some range of light radiation wavelengths -- color determined by wavelength distribut ...
Synthetic Polymers
... A second process results in the formation of shorter branches that contain only four carbons. These result when the radical end of a growing polymer chain reaches back and abstracts a hydrogen from itself. Because the cyclic transition state for this abstraction is most favorable when it contains si ...
... A second process results in the formation of shorter branches that contain only four carbons. These result when the radical end of a growing polymer chain reaches back and abstracts a hydrogen from itself. Because the cyclic transition state for this abstraction is most favorable when it contains si ...
Photopolymer
A photopolymer is a polymer that changes its properties when exposed to light, often in the ultraviolet or visible region of the electromagnetic spectrum. These changes are often manifested structurally, for example hardening of the material occurs as a result of cross-linking when exposed to light. An example is shown below depicting a mixture of monomers, oligomers, and photoinitiators that conform into a hardened polymeric material through a process called curing,.A wide variety of technologically useful applications rely on photopolymers, for example some enamels and varnishes depend on photopolymer formulation for proper hardening upon exposure to light. In some instances, an enamel can cure in a fraction of a second when exposed to light, as opposed to thermally cured enamels which can require half an hour or longer. Curable materials are widely used for medical, printing, and photoresist technologies. Changes in structural and chemical properties can be induced internally by chromophores that the polymer subunit already possesses, or externally by addition of photosensitive molecules. Typically a photopolymer consists of a mixture of multifunctional monomers and oligomers in order to achieve the desired physical properties, and therefore a wide variety of monomers and oligomers have been developed that can polymerize in the presence of light either through internal or external initiation. Photopolymers undergo a process called curing, where oligomers are cross-linked upon exposure to light, forming what is known as a network polymer. The result of photo curing is the formation of a thermoset network of polymers. One of the advantages of photo-curing is that it can be done selectively using high energy light sources, for example lasers, however, most systems are not readily activated by light, and in this case a photoinitiator is required. Photoinitiators are compounds that upon radiation of light decompose into reactive species that activate polymerization of specific functional groups on the oligomers. An example of a mixture that undergoes cross-linking when exposed to light is shown below. The mixture consists of monomeric styrene and oligomeric acrylates.Most commonly, photopolymerized systems are typically cured through UV radiation, since ultraviolet light is more energetic; however, the development of dye-based photoinitiator systems have allowed for the use of visible light, having potential advantages of processes that are more simple and safe to handle. UV curing in industrial processes has greatly expanded over the past several decades. Many traditional thermally cured and solvent-based technologies can be replaced by photopolymerization technologies. The advantages of photopolymerization over thermally cured polymerization include high rates of polymerization and environmental benefits from elimination of volatile organic solvents.There are two general routes for photoinitiation: free radical and ionic. The general process involves doping a batch of neat polymer with small amounts of photoinitiator, followed by selective radiation of light, resulting a highly cross-linked product. Many of these reactions do not require solvent which eliminates termination path via reaction of initiators with solvent and impurities, in addition to decreasing the overall cost.