FINAL REVIEW 1st SEMESTER 2014-2015
... 9. Find the empirical formula for the compound that contains 1.24 g Cr and .76 g O. ...
... 9. Find the empirical formula for the compound that contains 1.24 g Cr and .76 g O. ...
4.7 The two atoms containing molecule
... to the Coulomb interaction between the electrons are not understood until now, but they are most probably the reason for the unexpected high transition temperatures in these superconductors. To get a feeling for these problems and the strong coupling between magnetic properties and the Coulomb inter ...
... to the Coulomb interaction between the electrons are not understood until now, but they are most probably the reason for the unexpected high transition temperatures in these superconductors. To get a feeling for these problems and the strong coupling between magnetic properties and the Coulomb inter ...
Chapter 10 - Lecture 1
... 1) The exponential ensures that R(r) → 0 at large r 2) The ρl ensures that R(r) → 0 at the nucleus 3) The associated Laguerre polynomial oscillates from positive to negative and accounts for the radial nodes ...
... 1) The exponential ensures that R(r) → 0 at large r 2) The ρl ensures that R(r) → 0 at the nucleus 3) The associated Laguerre polynomial oscillates from positive to negative and accounts for the radial nodes ...
atomic currency
... ATOMIC CURRENCY I. Electrons A. Energy Levels - describes the area or region where an eis most likely to be found ...
... ATOMIC CURRENCY I. Electrons A. Energy Levels - describes the area or region where an eis most likely to be found ...
Atomic and Molecular Spectroscopy
... o Review of basic spectroscopy o Hydrogen energy levels o Fine structure o Spin-orbit coupling o Nuclear moments and hyperfine structure ...
... o Review of basic spectroscopy o Hydrogen energy levels o Fine structure o Spin-orbit coupling o Nuclear moments and hyperfine structure ...
Vocabulary:
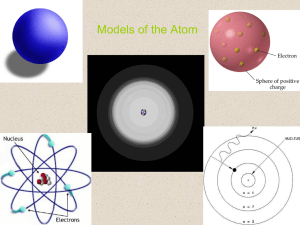
... Bohr’s Atomic Model Planetary System Model – Electrons move around the nucleus of an atom, like the planets around the sun. James Maxwell – Proposed that visible light consists of electromagnetic waves. Maxwell Planck – Suggested that atoms and molecules emit energy in discrete quantities, called qu ...
... Bohr’s Atomic Model Planetary System Model – Electrons move around the nucleus of an atom, like the planets around the sun. James Maxwell – Proposed that visible light consists of electromagnetic waves. Maxwell Planck – Suggested that atoms and molecules emit energy in discrete quantities, called qu ...
Electronic Structure and the Periodic Table A. Bohr Model of the
... A. Bohr Model of the Atom 1. Solar System Model 2. Created to Fit a “Quantized” Picture of Energy Transfer 3. Basis: Noncontinuous Emission Spectra of the Elements 4. Basic Postulates a. Electrons reside in certain allowed energy states b. Energy absorption and emission by atoms is caused by electro ...
... A. Bohr Model of the Atom 1. Solar System Model 2. Created to Fit a “Quantized” Picture of Energy Transfer 3. Basis: Noncontinuous Emission Spectra of the Elements 4. Basic Postulates a. Electrons reside in certain allowed energy states b. Energy absorption and emission by atoms is caused by electro ...
D NAME: 1. What is the eigenvalue of Lz for Ψ if the eigenval
... What is the name of the general class of functions "ar 2 ? How many nodes does this function have over r? ! represented by e ...
... What is the name of the general class of functions "ar 2 ? How many nodes does this function have over r? ! represented by e ...
Chapter 5 Electrons In Atoms 5.1 Models of the Atom The
... The model for the atom consisted of protons and neutrons making up a nucleus surrounded by electrons. The Bohr Model Bohr proposed that an ______________ is found only in specific circular paths, or orbits, around the nucleus. The fixed energies an electron can have are called __________________ lev ...
... The model for the atom consisted of protons and neutrons making up a nucleus surrounded by electrons. The Bohr Model Bohr proposed that an ______________ is found only in specific circular paths, or orbits, around the nucleus. The fixed energies an electron can have are called __________________ lev ...
AP Chemistry Chapter 6 Outline for Concepts to Know 6.1 Wave
... Line spectra for other elements are generally more complex, but are all due to various energy transitions of electrons moving from excited to ground (or less excited) levels Calculations of energy states of hydrogen and other atoms will NOT be tested Bohr model will not be specifically tested ...
... Line spectra for other elements are generally more complex, but are all due to various energy transitions of electrons moving from excited to ground (or less excited) levels Calculations of energy states of hydrogen and other atoms will NOT be tested Bohr model will not be specifically tested ...
Ch. 5.1 Models of the Atom
... The Quantum Mechanical Model Experimental results were inconsistent with the idea of electrons moving like large objects in orbit. Schrodinger proposed the quantum mechanical model. It does not involve the exact path an electron takes around the nucleus. ...
... The Quantum Mechanical Model Experimental results were inconsistent with the idea of electrons moving like large objects in orbit. Schrodinger proposed the quantum mechanical model. It does not involve the exact path an electron takes around the nucleus. ...
The Atom
... ground state quantumanumber De Broglie equation Heisenberg uncertainty principle quantum mechanical model of the atom atomic orbital principal quantum number principal energy level energy sublevel electron configuration Aufbau principle Hund’s Rule ...
... ground state quantumanumber De Broglie equation Heisenberg uncertainty principle quantum mechanical model of the atom atomic orbital principal quantum number principal energy level energy sublevel electron configuration Aufbau principle Hund’s Rule ...
Rutherford–Bohr model
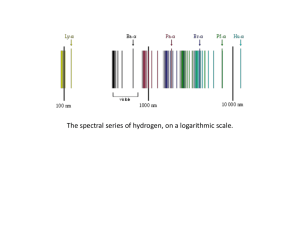
... atomic nucleus and where an electron jump between orbits is accompanied by an emitted or absorbed amount of electromagnetic energy (hν).[1] The orbits in which the electron may travel are shown as grey circles; their radius increases as n2, where n is the principal quantum number. The 3 → 2 transiti ...
... atomic nucleus and where an electron jump between orbits is accompanied by an emitted or absorbed amount of electromagnetic energy (hν).[1] The orbits in which the electron may travel are shown as grey circles; their radius increases as n2, where n is the principal quantum number. The 3 → 2 transiti ...