R - University of St Andrews
... Explanation: each energy level actually consists of several distinct states with almost the same energy. The first theory that justified this was done by Wilson and Sommerfeld: they conjectured that electron orbits can be elliptical, of which a circular orbit is a special case. Each orbit is specifi ...
... Explanation: each energy level actually consists of several distinct states with almost the same energy. The first theory that justified this was done by Wilson and Sommerfeld: they conjectured that electron orbits can be elliptical, of which a circular orbit is a special case. Each orbit is specifi ...
energy levels
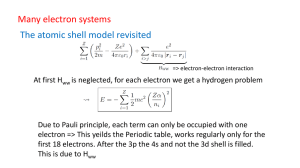
... – Tells you how far away the electron is from the nucleus – There are 1-7 energy levels, correlates with period numbers – Each level has same n number of sublevels – Maximum number of 2n2 electrons per level ...
... – Tells you how far away the electron is from the nucleus – There are 1-7 energy levels, correlates with period numbers – Each level has same n number of sublevels – Maximum number of 2n2 electrons per level ...
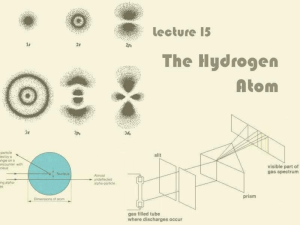
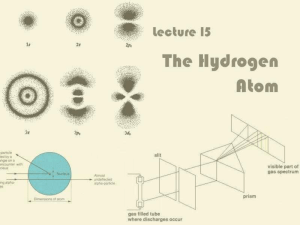
Lecture 15: The Hydrogen Atom
... For historical reasons, ℓ = 0, 1, 2, 3 is also known as s, p, d, f ...
... For historical reasons, ℓ = 0, 1, 2, 3 is also known as s, p, d, f ...
Chp 5 Guided Reading Notes and Vocabulary
... 9. Circle the letter of the formula for the maximum number of electrons that can occupy a principle energy level? Use n for the principle quantum number. a. 2n2 b. n2 c. 2n d. n 5.2 Electron Arrangement in Atoms ...
... 9. Circle the letter of the formula for the maximum number of electrons that can occupy a principle energy level? Use n for the principle quantum number. a. 2n2 b. n2 c. 2n d. n 5.2 Electron Arrangement in Atoms ...
Lecture 15: The Hydrogen Atom
... For historical reasons, ℓ = 0, 1, 2, 3 is also known as s, p, d, f ...
... For historical reasons, ℓ = 0, 1, 2, 3 is also known as s, p, d, f ...
Name
... Fission splits a large nucleus into smaller nuclei. Fusion combines two small nuclei into one larger one. 41. Briefly describe what happens that allows you to see colors in the flame tests and the gas tubes. When energy is added to an atom, an electron jumps to a higher energy level (excited state). ...
... Fission splits a large nucleus into smaller nuclei. Fusion combines two small nuclei into one larger one. 41. Briefly describe what happens that allows you to see colors in the flame tests and the gas tubes. When energy is added to an atom, an electron jumps to a higher energy level (excited state). ...
Isotopes and relative weight review sheet
... electron being located in it. _____g. Suggested that electrons orbit the nucleus in fixed paths with quantized energy levels. _____h. Experimented with cathode rays and discovered the existence of the electron. Elements and Their Isotopes Part of Atom ...
... electron being located in it. _____g. Suggested that electrons orbit the nucleus in fixed paths with quantized energy levels. _____h. Experimented with cathode rays and discovered the existence of the electron. Elements and Their Isotopes Part of Atom ...
Tuesday Aug 19
... atom’s electrons be found? 2. How do electrons become “excited”? 3. What form of energy emission accompanies the return of excited electrons to the ground state? 4. Assume that an atom has a total of four possible energy levels and that an electron can jump up or down between any of these energy lev ...
... atom’s electrons be found? 2. How do electrons become “excited”? 3. What form of energy emission accompanies the return of excited electrons to the ground state? 4. Assume that an atom has a total of four possible energy levels and that an electron can jump up or down between any of these energy lev ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 25. (a) State and illustrate the Pauli Exclusion Principle for the ground state of He atom. (b) Write the four Slater determinants for the excited state of He (1s1, 2s1). (7+3) 26. a) What are the three important approximations that distinguish the Huckel MO method from other LCAO methods? b) Write ...
... 25. (a) State and illustrate the Pauli Exclusion Principle for the ground state of He atom. (b) Write the four Slater determinants for the excited state of He (1s1, 2s1). (7+3) 26. a) What are the three important approximations that distinguish the Huckel MO method from other LCAO methods? b) Write ...
Atomic Structure and Quantum Theory
... the energy is not continuous but rather quantized… quantum mechanics was born ...
... the energy is not continuous but rather quantized… quantum mechanics was born ...
study note 1 06
... What name has been given to the current theory of electronic Wave or Quantum mechanics (because it is based on a wave model of structure? matter that predicts quantized energy levels). What two kinds of waves exist? Standing and traveling. Give an example of a standing wave. Guitar string, electron. ...
... What name has been given to the current theory of electronic Wave or Quantum mechanics (because it is based on a wave model of structure? matter that predicts quantized energy levels). What two kinds of waves exist? Standing and traveling. Give an example of a standing wave. Guitar string, electron. ...
Electronic Structure of Atoms (i.e., Quantum Mechanics)
... A quantum strikes a metal atom and the energy is absorbed by an e-. If the energy is sufficient, e- will leave its orbital, causing a current to flow throughout the ...
... A quantum strikes a metal atom and the energy is absorbed by an e-. If the energy is sufficient, e- will leave its orbital, causing a current to flow throughout the ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034 /1.00-4.00
... Part-C ANSWER ANY FOUR QUESTIONS (4 x 10 = 40) 23. (a) Write the Schroedinger equation to be solved for H atom and solve it for its energy using a simple solution, which assumes the wave function to depend only on the distance r and not on the angles θ and φ (b) In the Compton experiment, a beam of ...
... Part-C ANSWER ANY FOUR QUESTIONS (4 x 10 = 40) 23. (a) Write the Schroedinger equation to be solved for H atom and solve it for its energy using a simple solution, which assumes the wave function to depend only on the distance r and not on the angles θ and φ (b) In the Compton experiment, a beam of ...