Chemistry 2000 Review: quantum mechanics of
... This equation was know to belong to a special class known as an eigenvector equation: an operator acts on a function (ψ) and generates a scalar times the same function Ψ is known as the wavefunction of the electron: there are an infinite number of such wavefunctions, each of which is characterized b ...
... This equation was know to belong to a special class known as an eigenvector equation: an operator acts on a function (ψ) and generates a scalar times the same function Ψ is known as the wavefunction of the electron: there are an infinite number of such wavefunctions, each of which is characterized b ...
Modern physics 2330
... 3- ( ) The photoelectric effect takes place only if the energy of the incident electrons exceeds the energy of the photons. 4- ( ) X-rays are produced by bombarding a metal target with energetic electrons having energies of 50 to 100 KeV. 5- (..) A black hole is an object of sufficiently high densit ...
... 3- ( ) The photoelectric effect takes place only if the energy of the incident electrons exceeds the energy of the photons. 4- ( ) X-rays are produced by bombarding a metal target with energetic electrons having energies of 50 to 100 KeV. 5- (..) A black hole is an object of sufficiently high densit ...
Matter and Energy Identify a chemical physical change Identify a
... Ground and excited state Sublevels s p d f ...
... Ground and excited state Sublevels s p d f ...
ppt
... If level is N-fold degenerate (for example, p level is 3 fold degenerate) then it can accomondate 2N electrons. So we can now fill various atomic shells with electrons E1s2,E2s2E2p6 and so on until we accomodate all N electrons within various slots. Thus we obtain a periodic table. i.For atom with g ...
... If level is N-fold degenerate (for example, p level is 3 fold degenerate) then it can accomondate 2N electrons. So we can now fill various atomic shells with electrons E1s2,E2s2E2p6 and so on until we accomodate all N electrons within various slots. Thus we obtain a periodic table. i.For atom with g ...
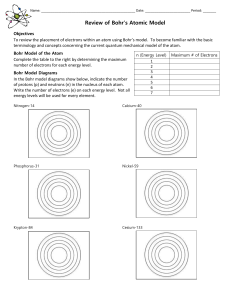
Name: ______ Date: Period: ______ Review of Bohr`s Atomic Model
... In the Bohr model diagrams show below, indicate the number of protons (p) and neutrons (n) in the nucleus of each atom. Write the number of electrons (e) on each energy level. Not all energy levels will be used for every element. ...
... In the Bohr model diagrams show below, indicate the number of protons (p) and neutrons (n) in the nucleus of each atom. Write the number of electrons (e) on each energy level. Not all energy levels will be used for every element. ...
Modern Physics Guide
... Interference pattern is a probability distribution for finding the quantum at the screen. Making a measurement collapses the wave function to that for the result. Uncertainty principle: ΔpΔx≥ħ ; ΔEΔt≥ħ due to the wave nature of the quantum. Pattern of wave functions (like standing waves), shape and ...
... Interference pattern is a probability distribution for finding the quantum at the screen. Making a measurement collapses the wave function to that for the result. Uncertainty principle: ΔpΔx≥ħ ; ΔEΔt≥ħ due to the wave nature of the quantum. Pattern of wave functions (like standing waves), shape and ...
Chemistry 1 Concept 5 “Electrons in Atoms” Study Guide
... 6. An electron for which n = 4 has more _______ than an electron for which n = 2. 7. What is the frequency of a photon with an energy of 1.75 x 10 -19 J________________ 8. The number of sublevels within each energy level of an atom is equal to the value of the _______________________________________ ...
... 6. An electron for which n = 4 has more _______ than an electron for which n = 2. 7. What is the frequency of a photon with an energy of 1.75 x 10 -19 J________________ 8. The number of sublevels within each energy level of an atom is equal to the value of the _______________________________________ ...
Quantum Mechanics
... Treating the orbit of an electron as a continuous wave, the path length (2πr) must be equal to a whole number of wavelengths ...
... Treating the orbit of an electron as a continuous wave, the path length (2πr) must be equal to a whole number of wavelengths ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI-600034 M.Sc. Part-A NOVEMBER 2015
... 24a. Derive the wave function and energy for a particle in a rectangular three dimensional box. b. Determine the wave length of light absorbed when an electron in a linear molecule of 11.8 Å long makes a transition from the energy level, n = 1 to n = 2. ...
... 24a. Derive the wave function and energy for a particle in a rectangular three dimensional box. b. Determine the wave length of light absorbed when an electron in a linear molecule of 11.8 Å long makes a transition from the energy level, n = 1 to n = 2. ...
3.3 Why do atoms radiate light?
... description they would always radiate light and thus be destroyed). This classical explanation results from the wrong picture, that the electron is moving through the orbital, leading to a steady change in the dipole moment. • Each state, which is not an Eigenstate of the Hamiltonian has a non infin ...
... description they would always radiate light and thus be destroyed). This classical explanation results from the wrong picture, that the electron is moving through the orbital, leading to a steady change in the dipole moment. • Each state, which is not an Eigenstate of the Hamiltonian has a non infin ...
Prentice Hall Chemistry Worksheets
... 1. The lowest-energy arrangement of electrons in a subshell is obtained by putting electrons into separate orbitals of the subshell before pairing electrons. ...
... 1. The lowest-energy arrangement of electrons in a subshell is obtained by putting electrons into separate orbitals of the subshell before pairing electrons. ...
5 ELECTRONS IN ATOMS Vocabulary Review Name ___________________________
... 1. The lowest-energy arrangement of electrons in a subshell is obtained by putting electrons into separate orbitals of the subshell before pairing electrons. ...
... 1. The lowest-energy arrangement of electrons in a subshell is obtained by putting electrons into separate orbitals of the subshell before pairing electrons. ...
Quantum Mechanical Model of the Atom
... spectra of any other element. Showed that an atom has Data indicates that quantized (specific) electrons don’t move energy levels. around nucleus in circular orbits. ...
... spectra of any other element. Showed that an atom has Data indicates that quantized (specific) electrons don’t move energy levels. around nucleus in circular orbits. ...
Ch.5 VocabReview
... 1. The lowest-energy arrangement of electrons in a subshell is obtained by putting electrons into separate orbitals of the subshell before pairing electrons. ...
... 1. The lowest-energy arrangement of electrons in a subshell is obtained by putting electrons into separate orbitals of the subshell before pairing electrons. ...
SG2 Atoms and Atomic Structure
... 3) Explain the further development of atomic theory a) Three subatomic particles (proton, neutron, electron): Know their names, location in the atom, charge, relative mass (in amu) b) Explain the octet rule and ion formation c) Use periodic table to identify atomic number and average atomic mass d) ...
... 3) Explain the further development of atomic theory a) Three subatomic particles (proton, neutron, electron): Know their names, location in the atom, charge, relative mass (in amu) b) Explain the octet rule and ion formation c) Use periodic table to identify atomic number and average atomic mass d) ...
c
... 1. Explain how the nucleus stays together despite the fact that it consists of positively charged particle and that like charged particles repel each other. 2. Explain the process of elemental transmutation and identify 2 ways in which this happens 3. Describe how average atomic mass is calculated w ...
... 1. Explain how the nucleus stays together despite the fact that it consists of positively charged particle and that like charged particles repel each other. 2. Explain the process of elemental transmutation and identify 2 ways in which this happens 3. Describe how average atomic mass is calculated w ...











![Chapter7_1 - Department of Chemistry [FSU]](http://s1.studyres.com/store/data/016128835_1-aea3c1aec04363d6cbf538e8faf80e45-300x300.png)