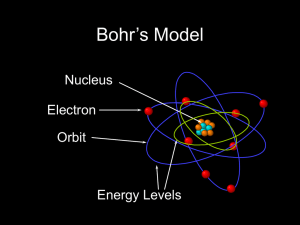
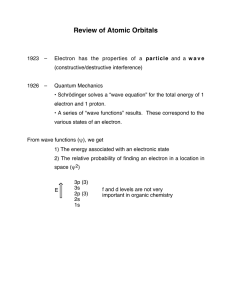
DEVELOPMENT OF THE ATOMIC THEORY PROJECT due Friday
... DEVELOPMENT OF THE ATOMIC THEORY PROJECT due Friday, Sept. 30 ...
... DEVELOPMENT OF THE ATOMIC THEORY PROJECT due Friday, Sept. 30 ...
Ch1-8 Brown and LeMay Review
... A) Can be used to predict that a gaseous carbon atom in its ground state is paramagnetic. B) Explains electron diffraction. C) Indicates that atomic orbitals can hold no more than two electrons. D) Predicts that it is impossible to determine simultaneously the exact position and exact velocity of an ...
... A) Can be used to predict that a gaseous carbon atom in its ground state is paramagnetic. B) Explains electron diffraction. C) Indicates that atomic orbitals can hold no more than two electrons. D) Predicts that it is impossible to determine simultaneously the exact position and exact velocity of an ...
1to7
... It didn’t explain such things as why elements give off light of specific colors when heated: Example: fireworks, iron ...
... It didn’t explain such things as why elements give off light of specific colors when heated: Example: fireworks, iron ...
Review Sheet
... Endothermic vs. exothermic Stoichiometry using energy (using enthalpy of reaction and balanced chemical reactions) Calculating H using Hess’s Law, Enthalpy Diagrams, and/or H° of formations Calorimetry calculations to determine H and q Standard States and enthalpy of formation Using Hess’s Law eq ...
... Endothermic vs. exothermic Stoichiometry using energy (using enthalpy of reaction and balanced chemical reactions) Calculating H using Hess’s Law, Enthalpy Diagrams, and/or H° of formations Calorimetry calculations to determine H and q Standard States and enthalpy of formation Using Hess’s Law eq ...
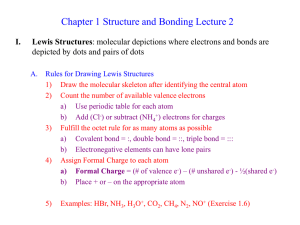
Ch 1 Lecture 2
... 2) Count the number of available valence electrons a) Use periodic table for each atom b) Add (Cl-) or subtract (NH4+) electrons for charges 3) Fulfill the octet rule for as many atoms as possible a) Covalent bond = :, double bond = ::, triple bond = ::: b) Electronegative elements can have lone pai ...
... 2) Count the number of available valence electrons a) Use periodic table for each atom b) Add (Cl-) or subtract (NH4+) electrons for charges 3) Fulfill the octet rule for as many atoms as possible a) Covalent bond = :, double bond = ::, triple bond = ::: b) Electronegative elements can have lone pai ...
Chapter 3
... a. The 4 quantum numbers and what they describe b. The difference between orbits (Bohr) and orbitals c. Pauli’s exclusion principle (no two electrons have the same 4 quantum #s) 3. Electron Configuration: a. Aufbau principle (add electrons to lowest energy orbitals first) b. Hund’s rule (1 electron ...
... a. The 4 quantum numbers and what they describe b. The difference between orbits (Bohr) and orbitals c. Pauli’s exclusion principle (no two electrons have the same 4 quantum #s) 3. Electron Configuration: a. Aufbau principle (add electrons to lowest energy orbitals first) b. Hund’s rule (1 electron ...
Test 1 Guide
... 13) The vaporization of liquid mercury is a example of a physical change. 14) The capacity to do work is called kinetic potential. 15) There are 20 mLs in 0.2 L. 16) Saltwater (after it is adequately filtered) is a good example of homogeneous mixture. 17) There are 3 significant figures in 0.00300 m ...
... 13) The vaporization of liquid mercury is a example of a physical change. 14) The capacity to do work is called kinetic potential. 15) There are 20 mLs in 0.2 L. 16) Saltwater (after it is adequately filtered) is a good example of homogeneous mixture. 17) There are 3 significant figures in 0.00300 m ...
Atomic structure ls on a periodic table.
... Atomic structure ls on a periodic table. -metals, and noble gases are found on a periodic table. Specify how to predict the charge expected for the most stable ion of an atom. the line spectra of atomic species relate to the idea of quantized states of electrons in atoms. A Jablonksi diagram may be ...
... Atomic structure ls on a periodic table. -metals, and noble gases are found on a periodic table. Specify how to predict the charge expected for the most stable ion of an atom. the line spectra of atomic species relate to the idea of quantized states of electrons in atoms. A Jablonksi diagram may be ...
Modern Model of the Atom Student Notes and Assignment
... Each energy sublevel corresponds to an ATOMIC ORBITAL (often referred to as a cloud) ...
... Each energy sublevel corresponds to an ATOMIC ORBITAL (often referred to as a cloud) ...
Introduction to Chemistry
... To describe the shapes of orbitals designated by s, p, d, and f and to discuss orbital energies. To define electron spin and the electron spin quantum number. To explain the Pauli exclusion principle. To show how the quantum mechanical model can be applied to atoms besides hydrogen. To trace the dev ...
... To describe the shapes of orbitals designated by s, p, d, and f and to discuss orbital energies. To define electron spin and the electron spin quantum number. To explain the Pauli exclusion principle. To show how the quantum mechanical model can be applied to atoms besides hydrogen. To trace the dev ...
Simple Harmonic Oscillator
... Never express yourself more clearly than you are able to think. Prediction is very difficult, especially about the future. - Niels Bohr ...
... Never express yourself more clearly than you are able to think. Prediction is very difficult, especially about the future. - Niels Bohr ...
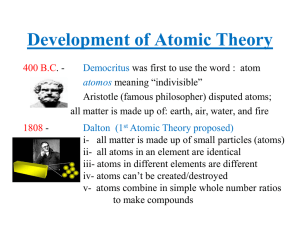
Development of Atomic Theory
... Heisenberg added to this concept that the position and velocity of an electron could never be simultaneously determined (Uncertainty Principle). All of these concepts/findings led to the development of our current model of the atom proposed by Schrodinger. ...
... Heisenberg added to this concept that the position and velocity of an electron could never be simultaneously determined (Uncertainty Principle). All of these concepts/findings led to the development of our current model of the atom proposed by Schrodinger. ...
Skill Assessment Sheet Modern Atomic Theory (Quantum Mechanics)
... I am approaching proficiency in meeting the standard. ( I could finish with some help) I can consistently demonstrate proficiency in meeting the standard. ( I can do this and will be able to do it later) I can consistently exceed key concepts, processes & skills. Exceeds the standard ( I can do more ...
... I am approaching proficiency in meeting the standard. ( I could finish with some help) I can consistently demonstrate proficiency in meeting the standard. ( I can do this and will be able to do it later) I can consistently exceed key concepts, processes & skills. Exceeds the standard ( I can do more ...
Semiclassical calculation of electron spectra in atoms through two
... The features of the one-electron spectra in the spherically symmetric self-consistent attraction potentials have been under study in the paper [1] (also, reviews [2, 3]). Specifically, the potentials with the Coulomb singularity have been there considered. As is well known, the screening of the Coul ...
... The features of the one-electron spectra in the spherically symmetric self-consistent attraction potentials have been under study in the paper [1] (also, reviews [2, 3]). Specifically, the potentials with the Coulomb singularity have been there considered. As is well known, the screening of the Coul ...
science 1 small-group tutorial scheme
... List all the subshells and orbitals for which the principal quantum number, n, has the values 2 and 3 in order of increasing energy, according to the quantum mechanical rules. Use clearly-labelled diagrams to illustrate the shapes and orientations of the orbitals for which the angular momentum (subs ...
... List all the subshells and orbitals for which the principal quantum number, n, has the values 2 and 3 in order of increasing energy, according to the quantum mechanical rules. Use clearly-labelled diagrams to illustrate the shapes and orientations of the orbitals for which the angular momentum (subs ...
Academic Chemistry Atomic History Study Guide 1. Identify and
... in World War I, contributed greatly to science prior to his death by discovering the atomic number. 11. According to the laws of classical physics, a charged particle traveling on a curved path will lose energy. This would cause an electron to drop into the nucleus, which would destroy the atom. ___ ...
... in World War I, contributed greatly to science prior to his death by discovering the atomic number. 11. According to the laws of classical physics, a charged particle traveling on a curved path will lose energy. This would cause an electron to drop into the nucleus, which would destroy the atom. ___ ...
Midterm TEKS Check Review 1. Define the following terms
... 28. Briefly describe what happens that allows you to see colors in the flame test lab. ...
... 28. Briefly describe what happens that allows you to see colors in the flame test lab. ...
Chap 2 Solns
... 2.4 (a) Two important quantum-mechanical concepts associated with the Bohr model of the atom are (1) that electrons are particles moving in discrete orbitals, and (2) electron energy is quantized into shells. (b) Two important refinements resulting from the wave-mechanical atomic model are (1) that ...
... 2.4 (a) Two important quantum-mechanical concepts associated with the Bohr model of the atom are (1) that electrons are particles moving in discrete orbitals, and (2) electron energy is quantized into shells. (b) Two important refinements resulting from the wave-mechanical atomic model are (1) that ...