The Quantum Mechanical Picture of the Atom
... required # of electrons starting from the lowest available energy level and following Pauli and Hund rules (this is called Aufbau principle) ...
... required # of electrons starting from the lowest available energy level and following Pauli and Hund rules (this is called Aufbau principle) ...
Problem Set 05
... a nitrogen molecule (N2) from its ground state (n=0) to the n=1 rotational state (for N2, d=0.11 nm). [You can check your result by noting that rotational spectra for small molecules are generally in the microwave region: 0.1mm→10cm]. ...
... a nitrogen molecule (N2) from its ground state (n=0) to the n=1 rotational state (for N2, d=0.11 nm). [You can check your result by noting that rotational spectra for small molecules are generally in the microwave region: 0.1mm→10cm]. ...
Chapter 6.8 - Periodic Trends
... electron in the Na atom would experience the pull of all 11 of its electrons. But this is not the case. The effective nuclear charge is less than 11 due to shielding effects. Which of the following statements correctly describe the effect of shielding? Select all that apply. a. Electrons closer to t ...
... electron in the Na atom would experience the pull of all 11 of its electrons. But this is not the case. The effective nuclear charge is less than 11 due to shielding effects. Which of the following statements correctly describe the effect of shielding? Select all that apply. a. Electrons closer to t ...
Modern Physics
... from the nucleus for the hydrogen atom is 2 ao. Find the probability of finding the 1-s electron at a distance greater than 2 ao according to quantum mechanics. ...
... from the nucleus for the hydrogen atom is 2 ao. Find the probability of finding the 1-s electron at a distance greater than 2 ao according to quantum mechanics. ...
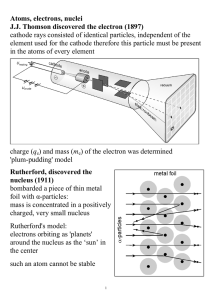
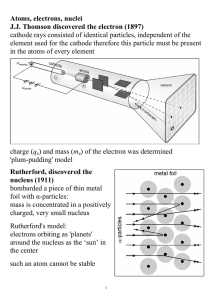
Atoms, electrons, nuclei J.J. Thomson discovered the electron (1897
... the less determined the momentum, and vice versa based on dimension analysis an uncertainty relation can be given for the case of energy · time: E t h. ...
... the less determined the momentum, and vice versa based on dimension analysis an uncertainty relation can be given for the case of energy · time: E t h. ...
Pre-AP Chemistry
... Focus on the following topics for the semester exam, being sure to relate terminology to concepts to calculations, as well as unifying principles between these topics. Questions will be focused on, but not limited to the specific items listed. Topics 1. science and chemistry 2. branches/courses in c ...
... Focus on the following topics for the semester exam, being sure to relate terminology to concepts to calculations, as well as unifying principles between these topics. Questions will be focused on, but not limited to the specific items listed. Topics 1. science and chemistry 2. branches/courses in c ...
The true nature of the atom?
... Yea, but is this quantum stuff really relevant? Does all this quantum stuff really matter outside of the classroom and lab? Yep, we’re about to look at the development of the quantum mechanical behavior of the atom, a theoretical framework that expresses the behavior of matter at the atomic scale. ...
... Yea, but is this quantum stuff really relevant? Does all this quantum stuff really matter outside of the classroom and lab? Yep, we’re about to look at the development of the quantum mechanical behavior of the atom, a theoretical framework that expresses the behavior of matter at the atomic scale. ...
Modern Atomic Theory Notes Sheet
... Bohr Model of the Atom Continued Said Hydrogen’s single electron could only be in certain allowed orbits around the nucleus higher orbit = theorized that electrons existed in distinct orbitals or energy levels around the nucleus, and it took an exact amount of energy or quanta to move an elec ...
... Bohr Model of the Atom Continued Said Hydrogen’s single electron could only be in certain allowed orbits around the nucleus higher orbit = theorized that electrons existed in distinct orbitals or energy levels around the nucleus, and it took an exact amount of energy or quanta to move an elec ...
Problem Set 1
... 1. According the Bohr atom model what is the speed of an electron for the ground state of Hydrogen atom? 2.Consider the absorption or emission of photon of energy hν by an atom initially at rest.After the transstion the atom has momentum P . If M is the mass of the atom find the frequency of the pho ...
... 1. According the Bohr atom model what is the speed of an electron for the ground state of Hydrogen atom? 2.Consider the absorption or emission of photon of energy hν by an atom initially at rest.After the transstion the atom has momentum P . If M is the mass of the atom find the frequency of the pho ...
Chapter 5 practice assessment
... Match the definition in Column A with the term in Column B. Column A ...
... Match the definition in Column A with the term in Column B. Column A ...
Arrangement of Electrons in Atoms
... Light is both wave and particle! Particle of light = photon, having zero mass and a quantum of energy Photons hit metal and knock e- out, but photon has to have enough energy ...
... Light is both wave and particle! Particle of light = photon, having zero mass and a quantum of energy Photons hit metal and knock e- out, but photon has to have enough energy ...