Help Sheet
... General rule: Any element with an odd-electron number must be paramagnetic. Shielding … describes why some orbitals are at higher or lower energy levels than others. Example: A 2s electron spends more of its time (on average) around the nucleus than a 2p electron due to the size of the orbitals. The ...
... General rule: Any element with an odd-electron number must be paramagnetic. Shielding … describes why some orbitals are at higher or lower energy levels than others. Example: A 2s electron spends more of its time (on average) around the nucleus than a 2p electron due to the size of the orbitals. The ...
Document
... Prove that, given a pair of normalized but not orthogonal functions ψ1 and ψ2, the function ψ3 = ψ2 – Sψ1 is orthogonal to ψ1 if S is the overlap integral of ψ1 and ψ2. Is ψ3 normalized? (Use the back of the page if necessary). ...
... Prove that, given a pair of normalized but not orthogonal functions ψ1 and ψ2, the function ψ3 = ψ2 – Sψ1 is orthogonal to ψ1 if S is the overlap integral of ψ1 and ψ2. Is ψ3 normalized? (Use the back of the page if necessary). ...
CHEMISTRY CHAPTER 5 OUTLINE NOTES 5.1 – Light and
... atomic model or Bohr’s Model. o So a hydrogen atom should be similar to a solar system consisting of a sun and one planet. o Scientists began to unravel the puzzle of chemical behavior in the early 1900’s and observed that certain elements emitted visible light when heated in a flame. o Analysis of ...
... atomic model or Bohr’s Model. o So a hydrogen atom should be similar to a solar system consisting of a sun and one planet. o Scientists began to unravel the puzzle of chemical behavior in the early 1900’s and observed that certain elements emitted visible light when heated in a flame. o Analysis of ...
Chapter 5 PPT/Notes A
... Parts of a Wave • Wavelength is the length of a wave from one location to the same location in the next wave…crest to crest for example. • Amplitude is the vertical distance from origin to crest or origin to trough. • The trough is the ‘bottom-point’ of a wave and the crest is the ‘peak’ of a wave. ...
... Parts of a Wave • Wavelength is the length of a wave from one location to the same location in the next wave…crest to crest for example. • Amplitude is the vertical distance from origin to crest or origin to trough. • The trough is the ‘bottom-point’ of a wave and the crest is the ‘peak’ of a wave. ...
MSE 221 Quantum Physics of Materials
... Coulomb Potential—Multi-electron atom, Hartree, many atom solid Pauli Principle—Antisymmetric eigenfunctions, covalent bonding in H2, F2, N2, O2, sp3 hydrids, Hund’s rule Molecular Orbitals Spectroscopies, Selection Rules Free Electron Theory—Drude classical theory, electrical and thermal conductivi ...
... Coulomb Potential—Multi-electron atom, Hartree, many atom solid Pauli Principle—Antisymmetric eigenfunctions, covalent bonding in H2, F2, N2, O2, sp3 hydrids, Hund’s rule Molecular Orbitals Spectroscopies, Selection Rules Free Electron Theory—Drude classical theory, electrical and thermal conductivi ...
Quantum Mechanical Model - Elmwood Park Memorial Middle School
... and velocity of extremely small particles at the same time Why? Think about how particles are detected or how your eyes work… ...
... and velocity of extremely small particles at the same time Why? Think about how particles are detected or how your eyes work… ...
Louie de Broglie
... It only estimates the probability of finding an electron in a certain position, unlike Bohr’s circular orbits. ...
... It only estimates the probability of finding an electron in a certain position, unlike Bohr’s circular orbits. ...
Superconcepts
... Quantum & electronic structure of the atom superconcepts This material is presented in chapter 6 of Brown et al. 12/e Superconcepts 1. An atom’s bonding & reactive properties are determined by electron configuration. 2. The behavior of electrons in atoms is dictated by quantum mechanics. Concepts a. ...
... Quantum & electronic structure of the atom superconcepts This material is presented in chapter 6 of Brown et al. 12/e Superconcepts 1. An atom’s bonding & reactive properties are determined by electron configuration. 2. The behavior of electrons in atoms is dictated by quantum mechanics. Concepts a. ...
PHYSICS 215 - Thermodynamics and Modern Physics Name:
... What is the minimum angle between L and the z axis? ...
... What is the minimum angle between L and the z axis? ...
CHAPTER 4 TEST REVIEW GUIDE
... Know how to use the Periodic Table to produce and read electron configurations. 12. Write electron configurations for selected elements in each of the three styles of notation (orbital diagram, complete electron configuration and noble gas notation). ...
... Know how to use the Periodic Table to produce and read electron configurations. 12. Write electron configurations for selected elements in each of the three styles of notation (orbital diagram, complete electron configuration and noble gas notation). ...
Quantum Mechanical Model
... Quantum Mechanical Model • As the energy of an electron increases, so does the quantum number (n) • Each principle energy level is also split up into one or more sublevels • Chart on Pg. 145 [http://www.chemistry.mcmaster.ca/esam/Chapter_4/fig4-2.jpg] ...
... Quantum Mechanical Model • As the energy of an electron increases, so does the quantum number (n) • Each principle energy level is also split up into one or more sublevels • Chart on Pg. 145 [http://www.chemistry.mcmaster.ca/esam/Chapter_4/fig4-2.jpg] ...
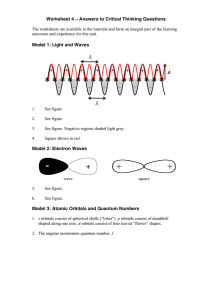
Answers to Critical Thinking Questions 4
... a. The Aufbau principle: Orbitals are filled with electrons in order of energy with the lowest energy orbital being filled first. b. Pauli Exclusion Principle: no two electrons may have the same set of quantum numbers. No orbital can accommodate more than 2 electrons. c. Hund’s Rule: in a set of orb ...
... a. The Aufbau principle: Orbitals are filled with electrons in order of energy with the lowest energy orbital being filled first. b. Pauli Exclusion Principle: no two electrons may have the same set of quantum numbers. No orbital can accommodate more than 2 electrons. c. Hund’s Rule: in a set of orb ...
Chapter 11 - Lecture 1
... 1. Mix at least 2 nonequivalent atomic orbitals (e.g. s and p). Hybrid orbitals have very different shape from original atomic orbitals. 2. Number of hybrid orbitals is equal to number of pure atomic orbitals used in the hybridization process. 3. Covalent bonds are formed by: a. Overlap of hybrid or ...
... 1. Mix at least 2 nonequivalent atomic orbitals (e.g. s and p). Hybrid orbitals have very different shape from original atomic orbitals. 2. Number of hybrid orbitals is equal to number of pure atomic orbitals used in the hybridization process. 3. Covalent bonds are formed by: a. Overlap of hybrid or ...
Atomic Structure and Periodicity
... They are held together by ionic bonds The Pauli exclusion principle states that no two electrons in the same atom can have identical quantum numbers It has the same number of protons and neutrons The number of electrons in the outermost shell determines the bonding characteristics of the element It ...
... They are held together by ionic bonds The Pauli exclusion principle states that no two electrons in the same atom can have identical quantum numbers It has the same number of protons and neutrons The number of electrons in the outermost shell determines the bonding characteristics of the element It ...