Lamb
... This formula implies a "rate" of transitions y for each atom of a collection of independent atoms. ...
... This formula implies a "rate" of transitions y for each atom of a collection of independent atoms. ...
stationary state
... • When an electron is in one of the quantized orbits, it does not emit any electromagnetic radiation; thus, the electron is said to be in a stationary state. • The electron can make a discontinuous emission, or quantum jump, from one stationary state to another. During this transition it does emit r ...
... • When an electron is in one of the quantized orbits, it does not emit any electromagnetic radiation; thus, the electron is said to be in a stationary state. • The electron can make a discontinuous emission, or quantum jump, from one stationary state to another. During this transition it does emit r ...
CHAPTER 5
... n – the principal quantum number As the orbital radius increases so does the energy (n-level) 1<2<3<4<5... ...
... n – the principal quantum number As the orbital radius increases so does the energy (n-level) 1<2<3<4<5... ...
Introduction to stat..
... • However all these summations are identical • If instead all states are different, to enumerate all allowed states we have only one choice, other permutations will represent identical states, therefore we need to divide by N! ...
... • However all these summations are identical • If instead all states are different, to enumerate all allowed states we have only one choice, other permutations will represent identical states, therefore we need to divide by N! ...
Pre-AP Chemistry
... 1. What kind of proportion is shown by the equation c = λ ν? 2. Who stated that electromagnetic radiation has a dual nature? 3. How many meters are 665 nm equal to? 4. What wave measure is identified by the unit Hz? 5. What wave property is known as distance between similar points on a wave? 6. What ...
... 1. What kind of proportion is shown by the equation c = λ ν? 2. Who stated that electromagnetic radiation has a dual nature? 3. How many meters are 665 nm equal to? 4. What wave measure is identified by the unit Hz? 5. What wave property is known as distance between similar points on a wave? 6. What ...
The Quantum Atom (section 18)
... electromagnetic radiation and lose energy. Why does the electron not do this? Excited gases emit line spectra: light at certain characteristic frequencies, not continuous spectra. They also absorb light only at these frequencies. Why? ...
... electromagnetic radiation and lose energy. Why does the electron not do this? Excited gases emit line spectra: light at certain characteristic frequencies, not continuous spectra. They also absorb light only at these frequencies. Why? ...
1.1 What has to be explained by Quantum mechanics?
... • Why are atoms stable? • Quantization: • ”Chemistry”: Period system of elements • sp3 hybridization • Spin • Band structure: Why is E(k) an adequate description of the energy of a crystal • Transport of electrons: from occupied state ⇒ free state Only reasonable for Fermions following the Pauli pri ...
... • Why are atoms stable? • Quantization: • ”Chemistry”: Period system of elements • sp3 hybridization • Spin • Band structure: Why is E(k) an adequate description of the energy of a crystal • Transport of electrons: from occupied state ⇒ free state Only reasonable for Fermions following the Pauli pri ...
Quantum Theory Historical Reference
... Charge of one mole of electrons = 96,485 C and is equivalent to 1F (faraday) 7. Ernest Rutherford(1871-1937): Discovered alpha () and beta () particles. Determined alpha particles to be positively charged. Gold foil experiment proved existence of positively charged and extremely dense nucleus surr ...
... Charge of one mole of electrons = 96,485 C and is equivalent to 1F (faraday) 7. Ernest Rutherford(1871-1937): Discovered alpha () and beta () particles. Determined alpha particles to be positively charged. Gold foil experiment proved existence of positively charged and extremely dense nucleus surr ...
24. The Helium Atom
... don’t exert any forces on each other, their combined wavefunction still needs to be antisymmetric under the hypothetical operation of interchanging them with each other. However, this combined wavefunction also includes their spin states. As we’ll study in a couple of weeks, the spin state alone, fo ...
... don’t exert any forces on each other, their combined wavefunction still needs to be antisymmetric under the hypothetical operation of interchanging them with each other. However, this combined wavefunction also includes their spin states. As we’ll study in a couple of weeks, the spin state alone, fo ...
1.3.4 Atoms and molecules Name Symbol Definition SI unit Notes
... see under Spectroscopy, section 3.5. ...
... see under Spectroscopy, section 3.5. ...
Elec Structure of Atom
... high energy state to a low energy state; light can be absorbed to excite the electron from a low energy state to a high energy state. The frequency of light emitted or absorbed must be such that hv=the difference in energy between two allowed states of the atom. ...
... high energy state to a low energy state; light can be absorbed to excite the electron from a low energy state to a high energy state. The frequency of light emitted or absorbed must be such that hv=the difference in energy between two allowed states of the atom. ...
Quantum Model Worksheet
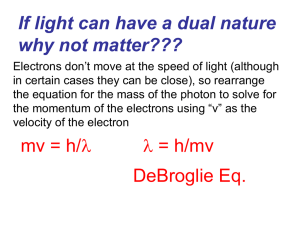
... Quantum Model of the Atom – Ch. 4 (p. 98 – 104) PART A – WAVES & QUANTUM MECHANICS 1. What experimental evidence supported de Broglie’s idea that electrons have wave-like properties? ...
... Quantum Model of the Atom – Ch. 4 (p. 98 – 104) PART A – WAVES & QUANTUM MECHANICS 1. What experimental evidence supported de Broglie’s idea that electrons have wave-like properties? ...